Chemistry:Hydrogen diselenide
From HandWiki
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dihydrogen diselenide
| |||
| Other names
Diselane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| 558110 | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| H2Se2 | |||
| Molar mass | 159.958 g·mol−1 | ||
| Appearance | oily liquid | ||
| Hazards | |||
| Flash point | Flammable | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tracking categories (test):
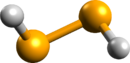
Hydrogen diselenide is an inorganic selenium compound with a chemical formula H
2Se
2 or (SeH)
2.[1][2] At room temperature, hydrogen diselenide dissociates easily to hydrogen selenide (H
2Se) and elemental selenium, and is therefore not stable. However, hydrogen diselenide can be stable in some solutions.[3]
References
- ↑ Macintyre, J.E. (1992). Dictionary of Inorganic Compounds. Taylor & Francis. pp. 293. ISBN 9780412301209.
- ↑ "化學命名原則". 國立編譯館. 2011-04-01. http://210.71.71.8/ischool/public/resource_view/open.php?file=77a6fd84d360a74163f9cb706cdf9618.pdf.
- ↑ Shaw, B.L.; Stavely, L.A.K. (2013). Inorganic Hydrides: The Commonwealth and International Library: Chemistry Division. Elsevier Science. pp. 76. ISBN 9781483160320.
 |