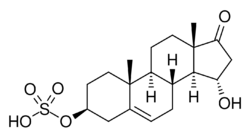
Chemistry:15α-Hydroxy-DHEA sulfate
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
15α-Hydroxy-17-oxoandrost-5-en-3β-yl hydrogen sulfate
| |
| Systematic IUPAC name
(3S,3aS,3bR,7S,9aR,9bS,11aS)-3-Hydroxy-9a,11a-dimethyl-1-oxo-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-7-yl hydrogen sulfate | |
| Other names
3β,15α-Dihydroxyandrost-5-en-17-one 3β-sulfate; 15α-Hydroxydehydroepiandrosterone sulfate; 15α-Hydroxy-DHEA sulfate; 15α-OH-DHEA-S
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| C19H28O6S | |
| Molar mass | 384.49 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
15α-Hydroxydehydroepiandrosterone sulfate, abbreviated as 15α-hydroxy-DHEA sulfate or 15α-OH-DHEA-S, also known as 15α-hydroxy-17-oxoandrost-5-en-3β-yl sulfate, is an endogenous, naturally occurring steroid and a metabolic intermediate in the production of estetrol from dehydroepiandrosterone (DHEA) during pregnancy.[1][2][3] It is the C3β sulfate ester of 15α-hydroxy-DHEA.[1][2][3]
See also
- Pregnenolone sulfate
- Dehydroepiandrosterone sulfate
- 16α-Hydroxydehydroepiandrosterone
- 16α-Hydroxyandrostenedione
- 16α-Hydroxyestrone
- Estrone sulfate
- C19H28O6S
References
- ↑ 1.0 1.1 Roger Smith (Prof.) (1 January 2001). The Endocrinology of Parturition: Basic Science and Clinical Application. Karger Medical and Scientific Publishers. pp. 91–. ISBN 978-3-8055-7195-1. https://books.google.com/books?id=0N6Ua0O8gWwC&pg=PA91.
- ↑ 2.0 2.1 J.B. Josimovich (11 November 2013). Gynecologic Endocrinology. Springer Science & Business Media. pp. 32–. ISBN 978-1-4613-2157-6. https://books.google.com/books?id=9vv2BwAAQBAJ&pg=PA32.
- ↑ 3.0 3.1 Jerome F. Strauss, III; Robert L. Barbieri (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. pp. 257–. ISBN 978-1-4557-2758-2. https://books.google.com/books?id=KZ95AAAAQBAJ&pg=PA257.
 |

