Chemistry:Einsteinium hexafluoride
From HandWiki
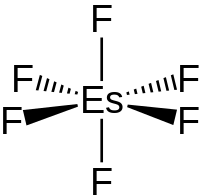
| |
| Names | |
|---|---|
| Other names
Einsteinium(VI) fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| EsF6 | |
| Molar mass | 366 g·mol−1 |
| Related compounds | |
Related compounds
|
Curium hexafluoride Americium hexafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Einsteinium hexafluoride is a binary inorganic chemical compound of einsteinium and fluorine with the chemical formula EsF
6. This is a hypothetical compound—its existence has been predicted theoretically, but the compound has yet to be isolated.[1][2][3][4]
Physical properties
It is unlikely that the compound is stable.[5]
References
- ↑ Macintyre, Jane E. (23 July 1992) (in en). Dictionary of Inorganic Compounds. CRC Press. p. 3121. ISBN 978-0-412-30120-9. https://books.google.com/books?id=9eJvoNCSCRMC&dq=Einsteinium+tetrafluoride&pg=PA3121. Retrieved 28 June 2023.
- ↑ Liebman, Joel F. (January 1978). "Conceptual problems in noble gas and fluorine chemistry, VII1: On the possible existence of einsteinium and proactinium hexafluoride". Inorganic and Nuclear Chemistry Letters 14 (6–7): 245–247. doi:10.1016/0020-1650(78)80069-5. https://www.sciencedirect.com/science/article/abs/pii/0020165078800695.
- ↑ Nishikida, Koichi; Williams, Ffrancon; Mamantov, Gleb; Smyrl, Norman (June 1975). "Chlorine hexafluoride radical. Preparation, electron spin resonance spectrum, and structure" (in en). Journal of the American Chemical Society 97 (12): 3526–3527. doi:10.1021/ja00845a046. ISSN 0002-7863. https://pubs.acs.org/doi/10.1021/ja00845a046. Retrieved 26 January 2024.
- ↑ Malta, John G.; Selig, Henry; Siegel, Stanley (January 1966). "Complex Compounds of Uranium Hexafluoride with Sodium Fluoride and Potassium Fluoride" (in en). Inorganic Chemistry 5 (1): 130–132. doi:10.1021/ic50035a031. ISSN 0020-1669. https://pubs.acs.org/doi/10.1021/ic50035a031. Retrieved 26 January 2024.
- ↑ Morss, L. R.; Edelstein, Norman M.; Fuger, Jean (21 October 2010) (in en). The Chemistry of the Actinide and Transactinide Elements (Set Vol.1-6): Volumes 1-6. Springer Science & Business Media. p. 1611. ISBN 978-94-007-0211-0. https://www.google.ru/books/edition/The_Chemistry_of_the_Actinide_and_Transa/9vPuV3A0UGUC?hl=en&gbpv=1&dq=Einsteinium+hexafluoride+EsF6&pg=PA1611&printsec=frontcover. Retrieved 26 January 2024.
 |

