Chemistry:Estradiol 17β-sulfate
From HandWiki
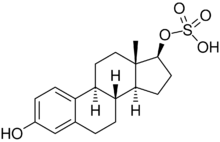
| |
| Names | |
|---|---|
| IUPAC name
3-Hydroxyestra-1,3,5(10)-trien-17β-yl hydrogen sulfate
| |
| Systematic IUPAC name
(1S,3aS,3bR,9bS,11aS)-7-Hydroxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthren-1-yl hydrogen sulfate | |
| Other names
Estra-1,3,5(10)-triene-3,17β-diol 17β-(hydrogen sulfate)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C18H24O5S | |
| Molar mass | 352.45 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Estradiol 17β-sulfate is an estrogen conjugate which is produced from estradiol by sulfation of the C17β hydroxyl group by estrogen sulfotransferases.[1][2][3][4]
See also
- Estradiol 3-sulfate
References
- ↑ "The physiological role of estradiol 17-sulfate during pregnancy". J. Steroid Biochem. Mol. Biol. 41 (3–8): 567–70. March 1992. doi:10.1016/0960-0760(92)90385-v. PMID 1314078.
- ↑ "[Endogenous levels and dynamics of estrogen sulfates--physiological and pathological roles of estrone sulfate and estradiol 17-sulfate]" (in ja). Nihon Naibunpi Gakkai Zasshi 68 (11): 1158–66. November 1992. doi:10.1507/endocrine1927.68.11_1158. PMID 1468592.
- ↑ "Sulfotransferase 2A1 forms estradiol-17-sulfate and celecoxib switches the dominant product from estradiol-3-sulfate to estradiol-17-sulfate". J. Steroid Biochem. Mol. Biol. 96 (5): 367–74. September 2005. doi:10.1016/j.jsbmb.2005.05.002. PMID 16011896.
- ↑ "Hepatic sulfation of estrogen metabolites". Biochim. Biophys. Acta 231 (1): 233–41. February 1971. doi:10.1016/0005-2760(71)90272-4. PMID 4323008.
 |

