Chemistry:Ruthenium(IV) fluoride
| |
| Names | |
|---|---|
| Other names
Ruthenium tetrafluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| F4Ru | |
| Molar mass | 177.06 g·mol−1 |
| Appearance | pink crystals |
| reacts with water | |
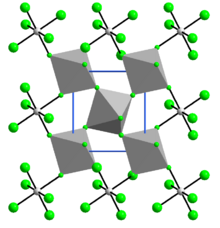
| Structure | |
| monoclinic | |
| Related compounds | |
Related compounds
|
Rhodium tetrafluoride, platinum tetrafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ruthenium(IV) fluoride is a binary inorganic compound of ruthenium and fluorine with the formula RuF
4.[1][2]
Synthesis
The compound was first prepared in 1963 by Holloway and Peacock, who obtained a yellow solid by reducing ruthenium pentafluoride with iodine, using iodine pentafluoride as a solvent.[3]
- 10RuF
5 + I
2 → 10RuF
4 + 2IF
5
- 10RuF
Subsequent studies have indicated that RuF4 produced by this way is impure. The pure, pink compound was isolated for the first time in 1992 by reacting KRuF6 with AsF5 at 20 °C in anhydrous hydrofluoric acid, with strict exclusion of water and oxygen. This synthesis exploits the very strong fluoride ion accepting capabilities of the Lewis acid AsF5.[4][5]
- K
2RuF
6 + 2AsF
5 → RuF
4 + 2KAsF
6
- K
Physical properties
RuF4 in the solid state is polymeric, with a three-dimensional structure of corrugated layers containing RuF
6 octahedra joined by shared fluorine atoms. The crystalline structure is similar to that of vanadium tetrafluoride and is monoclinic, space group P21/n, with lattice constants a = 560.7 pm, b = 494.6 pm, and c =514.3 pm, β = 121.27°.[6]
Ruthenium tetrafluoride is an extremely reactive compound which darkens immediately upon contact with moisture, and reacts violently with water to deposit ruthenium dioxide. The compound can be stored in glass containers, which are, however, attacked if the sample is heated above 280 °C.
References
- ↑ Casteel, William J.; Wilkinson, Angus P.; Borrmann, Horst; Serfass, Robert E.; Bartlett, Neil (July 1992). "Preparation and structure of ruthenium tetrafluoride and a structural comparison with ruthenium trifluoride and ruthenium pentafluoride" (in en). Inorganic Chemistry 31 (14): 3124–3131. doi:10.1021/ic00040a024. ISSN 0020-1669. https://pubs.acs.org/doi/10.1021/ic00040a024. Retrieved 10 April 2023.
- ↑ Bartlett, Neil (2001) (in en). The Oxidation of Oxygen and Related Chemistry: Selected Papers of Neil Bartlett. World Scientific. p. 306. ISBN 978-981-281-198-1. https://books.google.com/books?id=GbpR-Vuy0UcC&dq=Ruthenium+tetrafluoride&pg=PA273. Retrieved 10 April 2023.
- ↑ (in en) Reactor Fuel Processing. U.S. Argonne National Laboratory.. 1963. p. 28. https://books.google.com/books?id=5xzHE8cSNjMC&dq=Ruthenium+tetrafluoride&pg=RA11-PA28. Retrieved 10 April 2023.
- ↑ Casteel, William J.; Wilkinson, Angus P.; Borrmann, Horst; Serfass, Robert E.; Bartlett, Neil (July 1992). "Preparation and structure of ruthenium tetrafluoride and a structural comparison with ruthenium trifluoride and ruthenium pentafluoride" (in en). Inorganic Chemistry 31 (14): 3124–3131. doi:10.1021/ic00040a024. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic00040a024. Retrieved 10 April 2023.
- ↑ Esteban, G. L.; Kerr, J. A.; Trotman-Dickenson, A. F.; Gronowitz, S.; Katritzky, A. R.; Reavill, R. E.; Ridgewell, B. J.; Green, M. et al. (1 January 1963). "Notes" (in en). Journal of the Chemical Society (Resumed): 3879–3919. doi:10.1039/JR9630003879. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1963/JR/jr9630003879. Retrieved 10 April 2023.
- ↑ "WebElements Periodic Table » Ruthenium » ruthenium tetrafluoride". webelements.com. https://webelements.com/compounds/ruthenium/ruthenium_tetrafluoride.html.
 |

