Chemistry:2-Methoxyestriol
From HandWiki
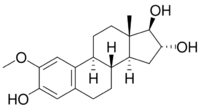
| |
| Names | |
|---|---|
| IUPAC name
2-Methoxyestra-1,3,5(10)-triene-3,16α,17β-triol
| |
| Systematic IUPAC name
(1R,2R,3aS,3bR,9bS,11aS)-8-Methoxy-11a-methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,2,7-triol | |
| Other names
2-MeO-E3
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C19H26O4 | |
| Molar mass | 318.413 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Methoxyestriol (2-MeO-E3) is an endogenous estrogen metabolite.[1][2][3] It is specifically a metabolite of estriol and 2-hydroxyestriol.[1][2][3] It has negligible affinity for the estrogen receptors and no estrogenic activity.[4] However, 2-methoxyestriol does have some non-estrogen receptor-mediated cholesterol-lowering effects.[5]
See also
References
- ↑ 1.0 1.1 "2-Methoxyestriol: a new metabolite of estradiol in man". Arch. Biochem. Biophys. 77 (2): 511–3. October 1958. doi:10.1016/0003-9861(58)90097-3. PMID 13584013.
- ↑ 2.0 2.1 "Oestriol metabolism by rat- and rabbit-liver slices. Isolation of 2-methoxyoestriol and 2-hydroxyestriol". Biochem. J. 79 (2): 355–61. May 1961. doi:10.1042/bj0790355. PMID 13756104.
- ↑ 3.0 3.1 "Radioimmunoassay of 2-methoxyestriol in pregnancy plasma". Horm. Metab. Res. 20 (9): 599–600. September 1988. doi:10.1055/s-2007-1010895. PMID 3198067.
- ↑ "The role of 2-methoxyestrone in estrogen action". J. Steroid Biochem. 19 (1B): 635–8. July 1983. doi:10.1016/0022-4731(83)90229-7. PMID 6310247.
- ↑ Kono, Shinzo; Higa, Hiroaki; Sunagawa, Hajime (1989). "Hypocholesterolemic Effect of Long-Term Continuous Administration of 2-Methoxyestriol in Dietary Hypercholesterolemic Rats". Journal of Clinical Biochemistry and Nutrition 6 (1): 49–56. doi:10.3164/jcbn.6.49. ISSN 1880-5086.
 |

