Chemistry:Cyclopentamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | N,α-dimethyl-cyclopenaneethylamine |
| Routes of administration | Topical (nasal spray) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
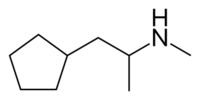
| Formula | C9H19N |
| Molar mass | 141.258 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Boiling point | 171 °C (340 °F) |
| |
| |
| | |
Cyclopentamine (trade names Clopane, Cyclonarol, Cyclosal, Cyklosan, Nazett, Sinos, among others) is a sympathomimetic alkylamine, classified as a vasoconstrictor. Cyclopentamine was indicated in the past as an over-the-counter (OTC) medication for use as a nasal decongestant, notably in Europe and Australia , but has now been largely discontinued.
Pharmacology
Cyclopentamine acts as a releasing agent of the catecholamine neurotransmitters norepinephrine (noradrenaline), epinephrine (adrenaline), and dopamine.[1] Its effects on norepinephrine and epinephrine mediate its decongestant effects, while its effects on all three neurotransmitters are responsible for its stimulant properties. When ingested orally in sufficient quantities, cyclopentamine produces similar effects to amphetamine, methamphetamine, and propylhexedrine.[2][3]
Chemistry
Cyclopentamine is the cyclopentane homolog of propylhexedrine, differing only in terms of the contracted ring size of a cyclopentane, containing one —CH2— unit less than the cyclohexyl group.
In terms of the acyclic part of the molecule, both cyclopentamine and propylhexedrine are the same as methamphetamine, all three molecules containing the 2-methylaminopropyl side-chain. The difference between them is that whereas methamphetamine is an aromatic molecule containing a phenyl group, cyclopentamine and propylhexedrine are entirely aliphatic and contain no delocalized electrons at all. The effect that this has on potency is that the reduced alicyclic-alkylamines are weaker than unsaturated (meth)amphetamine.[citation needed]
See also
- Amphetamine
- Cypenamine (which is trans-2-phenylcyclopentylamine)
- Methamphetamine
- Propylhexedrine (also known as cyclohexylisopropylmethylamine)
- Tranylcypromine (which is trans-2-phenylcyclopropylamine)
- Methiopropamine
References
- ↑ "A Nonsympathomimetic Effect of Cyclopentamine and Beta-Mercaptoethylamine in the Rabbit Ileum". The Journal of Pharmacology and Experimental Therapeutics 145: 83–6. July 1964. PMID 14209515.
- ↑ "In vitro evaluation of a series of sympathomimetic amines and the beta-adrenergic blocking properties of cyclopentamine". Journal of Pharmaceutical Sciences 58 (7): 882–4. July 1969. doi:10.1002/jps.2600580722. PMID 4390216.
- ↑ "Actions of dexamphetamine and amphetamine-like amines in chickens with brain transections". British Journal of Pharmacology 42 (4): 522–42. August 1971. doi:10.1111/j.1476-5381.1971.tb07138.x. PMID 5116035.
 |

