Chemistry:Levopropylhexedrine
From HandWiki
Short description: Chemical compound
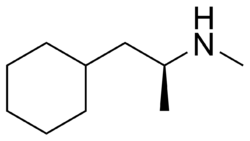 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C10H21N |
| Molar mass | 155.285 g·mol−1 |
| 3D model (JSmol) | |
| |
Levopropylhexedrine (Eventin) is an adrenergic alkylamine used as an anorectic in Germany [1][2] and patented by Smith Kline & French in 1947.[3] It has also been used in the anticonvulsant preparation barbexaclone in combination with phenobarbital to offset sedation.[4][5][6] Levopropylhexedrine is the levorotatory S-enantiomer of propylhexedrine. The dextrorotatory counterpart is known as dextropropylhexedrine.
Synthesis
The enantiopure synthesis of levopropylhexedrine may be accomplished in a two step reaction. The first step is the Wenker synthesis of the relevant aziridine. The second step is the simple catalytic hydrogenation of the propylhexedrine-aziridine.[7]
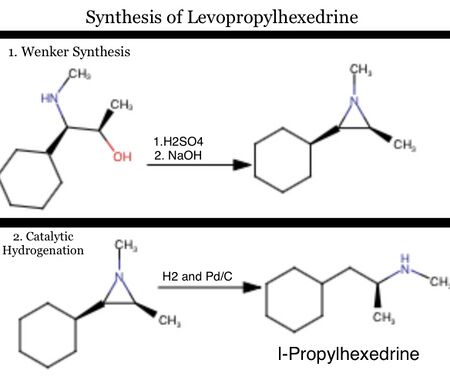
Selective synthesis of Levopropylhexedrine
See also
References
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA606.
- ↑ "Über therapeutische Erfahrungen mit dem Appetitzügler Eventin" (in de). Wiener Medizinische Wochenschrift 108 (14): 304–6. 1958. PMID 13558159.
- ↑ US 2454746 Cyclohexylalkylamines
- ↑ "Ensaio clínico com barbexaclone nas epilepsias" (in pt). Arquivos de Neuro-Psiquiatria 35 (1): 68–72. 1977. doi:10.1590/s0004-282x1977000100008. PMID 14606.
- ↑ "Pharmacokinetics of phenobarbital and propylhexedrine after administration of barbexaclone in the mouse". Naunyn-Schmiedeberg's Archives of Pharmacology 324 (2): 153–9. 1983. doi:10.1007/bf00497022. PMID 6139756.
- ↑ Seyffart, G. (1991). Drug dosage in renal insufficiency. Boston: Kluwer Academic Publishers. ISBN 0-7923-0964-2. https://books.google.com/books?id=FubgMz6aOWAC&pg=PA56.
- ↑ Haberl, R. (1958-11-01). "Über eine neue Bildungsweise des 1,2-Dimethyl-3-phenyl-äthylenimins" (in de). Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 89 (6): 814–816. doi:10.1007/BF00902540. ISSN 1434-4475. https://doi.org/10.1007/BF00902540.
 |

