Chemistry:Clobenzorex
 | |
| Clinical data | |
|---|---|
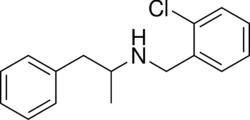
| Other names | N-(2-chlorobenzyl)amphetamine |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C16H18ClN |
| Molar mass | 259.78 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clobenzorex (Asenlix, Dinintel, Finedal, Rexigen) is a stimulant drug of the amphetamine chemical class used as an appetite suppressant.[2] The drug is legally distributed in Mexico under the trade name Asenlix by Aventis.
Chemically, clobenzorex is an N-substituted amphetamine prodrug that is metabolized primarily into 4-hydroxyclobenzorex after ingestion; however, small amounts are also metabolized into dextroamphetamine.[3] In commercial production, clobenzorex is supplied as the hydrochloride salt in green-tinted capsules. The drug gained use as a prescription anorectic in the 1970s.
Chemistry
Synthesis

Condensation between amphetamine (1) and 2-chlorobenzaldehyde (2) gives a Schiff-base, CID:135056236 (3). Subsequent reduction with sodium borohydride completed the synthesis of clobenzorex (4).
Apparently, also made from the acid chloride, and reduction of the amide with lithium aluminium anhydride.[citation needed]
Detection in urine
Clobenzorex can be detected in urine, which can cause false positives for workplace drug screening.[7] It is one of many drugs that can cause false positives for amphetamine urine drug screening.[8] It may be differentiated from amphetamine use through testing for metabolites such as 4-hydroxyclobenzorex[9] or enantiomeric analysis.[7]
Society and culture
Legal status
In Canada, Clobenzorex is not specifically listed in the CDSA, however due to structural similarities with norbenzphetamine, it is a schedule I under item 19(17).[citation needed]
In the UK it is a controlled drug (class B).[10] In Brazil it's a controlled prohibited psychotropic (class A3).[11]
The substance is not scheduled in the United States and is unaffected by the Federal Analogue Act as a derivative of Benzphetamine. Clobenzorex is legal in the United States of America. [12]
Clobenzorex is not controlled within the United States or subject to import controls. Importation of clobenzorex for personal use is lawful provided that is for use to treat a condition with no approved medications, unlawful marketing is not occurring in the U.S, not deemed hazardous to health for the treating the condition, and is verified as a continuation of a treatment plan that began in a foreign country.[13]
Recreational use
The use of clobenzorex is banned by the World Anti-Doping Agency for use during sports competitions.[14]
See also
References
- ↑ Boos, Terrence (April 6, 2023). "Clobenzorex Letter". https://imgur.io/MDUoDT4.
- ↑ "Clobenzorex: evidence for amphetamine-like behavioral actions". Pharmacology, Biochemistry, and Behavior 56 (2): 311–316. February 1997. doi:10.1016/s0091-3057(96)00329-2. PMID 9050090.
- ↑ "Amphetamines: methods of forensic analysis.". Handbook of Forensic Drug Analysis.. Elsevier. 2005. pp. 357–451 (430). ISBN 978-0-08-047289-8. https://books.google.com/books?id=8JsQgRO3QcwC&dq=Clobenzorex+metabolism&pg=PA430. "Amphetamine produced from the metabolism of clobenzorex has been shown to be the d-enantiomer only ..."
- ↑ "[New derivatives of phenylisopropylamine: synthesis and study of their anorexic activity]" (in French). Annales Pharmaceutiques Françaises 24 (1): 57–68. January 1966. PMID 5910702.
- ↑ "New substituted benzylamines and their salts and process for preparation" GB patent 1123565, issued 1968, assigned to Soc. Ind. Fabric. Antibiot.
- ↑ "Synthèse du chlorhydrate de (+)-N-(o-chlorobenzyl) α-methyl phénéthylamine marqué en position 7 par 14C (chlorhydrate de clobenzorex).". Journal of Labelled Compounds 6 (3): 289–297. July 1970. doi:10.1002/jlcr.2590060310.
- ↑ 7.0 7.1 (in en) Forensic Toxicology. Academic Press. 2018-01-02. pp. 245, 290. ISBN 978-0-12-800818-8. https://books.google.com/books?id=icS3CgAAQBAJ&dq=Clobenzorex+urine&pg=PA290.
- ↑ (in en) On Call Psychiatry E-Book: On Call Series. Elsevier Health Sciences. 2018-02-23. pp. 304. ISBN 978-0-323-54721-5. https://books.google.com/books?id=9vVNDwAAQBAJ&dq=Clobenzorex+urine&pg=PA304.
- ↑ "Amphetamine, Clobenzorex, and 4-Hydroxyclobenzorex Levels Following Multidose Administration of Clobenzorex" (in en). Journal of Analytical Toxicology 25 (3): 158–165. 2001-04-01. doi:10.1093/jat/25.3.158. ISSN 0146-4760. PMID 11327347.
- ↑ "Misuse of Drugs Act 1971 (c. 38): SCHEDULE 2: Controlled Drugs". Office of Public Sector Information. http://www.legislation.gov.uk/ukpga/1971/38/schedule/2.
- ↑ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" (in pt-BR). Diário Oficial da União. https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-804-de-24-de-julho-de-2023-498447451.
- ↑ Boos, Terrence (April 6, 2023). "Clobenzorex Letter". https://imgur.io/MDUoDT4.
- ↑ "Is it legal for me to personally import drugs?". Food and Drug Administration. 28 June 2021. https://www.fda.gov/about-fda/fda-basics/it-legal-me-personally-import-drugs.
- ↑ "World Anti-Doping Code International Standard Prohibited List 2023". September 2022. https://www.wada-ama.org/sites/default/files/2022-09/2023list_en_final_9_september_2022.pdf.
 |

