Chemistry:Aminorex
Aminorex, sold under the brand names Menocil and Apiquel among others, is a weight loss (anorectic) stimulant drug.[1][2] It was withdrawn from the market after it was found to cause pulmonary hypertension (PPH).[2][3] In the United States, aminorex is a Schedule I controlled substance.
Aminorex, in the 2-amino-5-aryloxazoline group, was developed by McNeil Laboratories in 1962.[4] It is closely related to 4-methylaminorex (4-MAR). Aminorex has been shown to have locomotor-stimulant effects, lying midway between dextroamphetamine and methamphetamine. Aminorex effects have been attributed to the release of catecholamines.[5] It can be produced as a metabolite of the deworming medication levamisole, which is sometimes used as a cutting agent of illicitly produced cocaine.[6][7]
Medical uses
Aminorex was formerly used as an appetite suppressant.[8]
Pharmacology
Pharmacodynamics
Aminorex is a serotonin–norepinephrine–dopamine releasing agent (SNDRA).[9][10][11] Its EC50 values for induction of monoamine release are 26.4 nM for norepinephrine, 49.4 nM for dopamine, and 193 nM for serotonin.[9][10][11] In addition to its monoamine-releasing activity, aminorex is a weak agonist of the serotonin 5-HT2 receptors, including of the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors.[10] Its EC50 values for activation of these receptors are 4,365 nM for 5-HT2A, 870 nM for 5-HT2B, and 525 nM for 5-HT2C.[10]
| Compound | NE | DA | 5-HT | Ref |
|---|---|---|---|---|
| Phenethylamine | 10.9 | 39.5 | >10,000 | [12][13][14] |
| Dextroamphetamine | 6.6–10.2 | 5.8–24.8 | 698–1,765 | [15][16][14][17] |
| Dextromethamphetamine | 12.3–14.3 | 8.5–40.4 | 736–1,292 | [15][18][14][17] |
| Aminorex | 15.1–26.4 | 9.1–49.4 | 193–414 | [15][19][14][8][17] |
| cis-4-MAR | 4.8 | 1.7 | 53.2 | [8][19] |
| cis-4,4'-DMAR | 11.8–31.6 | 8.6–24.4 | 17.7–59.9 | [19][20][8] |
| trans-4,4'-DMAR | 31.6 | 24.4 | 59.9 | [20][8] |
| cis-MDMAR | 14.8 | 10.2 | 43.9 | [20] |
| trans-MDMAR | 38.9 | 36.2 | 73.4 | [20] |
| Notes: The smaller the value, the more strongly the drug releases the neurotransmitter. The assays were done in rat brain synaptosomes and human potencies may be different. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. Refs:[21][10] | ||||
Activation of serotonin 5-HT2B receptors by aminorex, either directly via agonism or indirectly via serotonin release, has been implicated in the development of pulmonary arterial hypertension and cardiac valvulopathy with the drug.[10][9][22][11] However, its EC50 for serotonin 5-HT2B receptor activation is 33-fold higher than its EC50 value for induction of norepinephrine release and is almost 50-fold less potent than the serotonin 5-HT2B receptor agonism of dexnorfenfluramine.[10] This seems to call into question the role of direct agonism of the serotonin 5-HT2B receptor in the toxicity of aminorex.[10] Along similar lines, chlorphentermine, a related drug that has also been associated with such adverse effects, shows negligible direct serotonin 5-HT2B receptor agonistic activity.[10] However, it is possible that metabolites of aminorex and chlorphentermine might be more potent in this action.[10]
Aminorex does not appear to have been assessed at the trace amine-associated receptor 1 (TAAR1).[23][24] However, several derivatives of aminorex, such as 4-methylaminorex (4-MAR) and 4,4'-dimethylaminorex (4,4'-DMAR), have been found to be inactive at the mouse and rat TAAR1.[8][25][26] Many other monoamine releasing agents (MRAs), such as many amphetamines, are rodent and/or human TAAR1 agonists.[27][28] Activation of the TAAR1 may auto-inhibit and thereby constrain the monoaminergic effects of these agents.[8][25][26] Lack of TAAR1 agonism in the case of aminorex analogues might enhance their effects relative to MRAs possessing TAAR1 agonism.[8][25][26]
Chemistry
Aminorex is a member of the 2-amino-5-phenyloxazoline group.[1] It is structurally related to the substituted amphetamines like amphetamine and to the substituted phenylmorpholines like phenmetrazine.[1]
A variety of derivatives and analogues of aminorex are known.[1] These include 2'-fluoro-4-methylaminorex (2F-MAR), 2C-B-aminorex, 3',4'-methylenedioxy-4-methylaminorex (MDMAR), 4'-bromo-4-methylaminorex (4B-MAR), 4'-chloro-4-methylaminorex (4C-MAR), 4'-fluoro-4-methylaminorex (4F-MAR), 4-methylaminorex (4-MAR), 4,4'-dimethylaminorex (4,4'-DMAR), clominorex, cyclazodone, fenozolone, fluminorex, pemoline, and thozalinone, among others.[1][8][26]
Synthesis
The synthesis was first reported in a structure-activity relationship study of 2-amino-5-aryl-2-oxazolines, where aminorex was found to be approximately 2.5 times more potent than D-amphetamine sulfate in inducing anorexia in rats, and was also reported to have CNS stimulant effects.
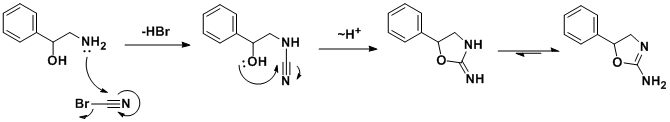
The racemic synthesis involves addition/cyclization reaction of 2-amino-1-phenylethanol with cyanogen bromide.[29] A similar synthesis has been also published.[30] In a search for a cheaper synthetic route, a German team developed an alternative route[31] which, by using chiral styrene oxide, allows an enantiopure product.
History
It was discovered in 1962 by Edward John Hurlburt,[32] and was quickly found in 1963 to have an anorectic effect in rats. It was introduced as a prescription appetite suppressant in Germany, Switzerland and Austria in 1965, but was withdrawn in 1972 after it was found to cause pulmonary hypertension in approximately 0.2% of patients, and was linked to a number of deaths.[5][33]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. 2014. p. 54. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA54. Retrieved 10 January 2025.
- ↑ 2.0 2.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Netherlands. 2012. p. 14. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA14. Retrieved 10 January 2025.
- ↑ "Recreational use of aminorex and pulmonary hypertension". Chest 118 (5): 1496–1497. November 2000. doi:10.1378/chest.118.5.1496. PMID 11083709. http://www.chestjournal.org/cgi/pmidlookup?view=long&pmid=11083709.
- ↑ Ireland PG, "2-Amino-5-Aryloxazoline Products", US patent 3161650, issued 15 December 1964
- ↑ 5.0 5.1 "Aminorex to fen/phen: an epidemic foretold". Circulation 99 (1): 156–161. Jan 1991. doi:10.1161/01.CIR.99.1.156. PMID 9884392.
- ↑ "Aminorex and rexamino as metabolites of levamisole in the horse". Analytica Chimica Acta 638 (1): 58–68. April 2009. doi:10.1016/j.aca.2009.02.033. PMID 19298880. Bibcode: 2009AcAC..638...58H.
- ↑ "Determination of aminorex in human urine samples by GC-MS after use of levamisole". Journal of Pharmaceutical and Biomedical Analysis 55 (5): 1186–1189. July 2011. doi:10.1016/j.jpba.2011.03.039. PMID 21531521.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 "DARK Classics in Chemical Neuroscience: Aminorex Analogues". ACS Chem Neurosci 9 (10): 2484–2502. October 2018. doi:10.1021/acschemneuro.8b00415. PMID 30269490. "Due to the lack of interaction with the trace amine-associated receptor 1 (TAAR1), 4,4'- DMAR is suspected to be unable to trigger the auto-inhibitory pathway that, for example, MDMA possesses at least in rodents135,183,184. [...] As mentioned before, in contrast to other amphetamine-type stimulants, 4,4'-DMAR does not interact with TAAR1 and therefore lacks the auto-inhibitory pathway that attenuates monoamine release and mediates the neuroprotective effects231,232. It has however been shown that many psychoactive compounds stimulate human TAAR1 less potently than the receptor’s rodent counterparts184.".
- ↑ 9.0 9.1 9.2 "Therapeutic and adverse actions of serotonin transporter substrates". Pharmacol Ther 95 (1): 73–88. July 2002. doi:10.1016/s0163-7258(02)00234-6. PMID 12163129.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 "Therapeutic potential of monoamine transporter substrates". Curr Top Med Chem 6 (17): 1845–1859. 2006. doi:10.2174/156802606778249766. PMID 17017961.
- ↑ 11.0 11.1 11.2 "Serotonin releasing agents. Neurochemical, therapeutic and adverse effects". Pharmacol Biochem Behav 71 (4): 825–836. April 2002. doi:10.1016/s0091-3057(01)00669-4. PMID 11888573.
- ↑ "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug and Alcohol Dependence 147: 1–19. February 2015. doi:10.1016/j.drugalcdep.2014.12.005. PMID 25548026.
- ↑ Synthesis and Biological Evaluation of Rigid Analogues of Methamphetamines. 22 May 2012. https://scholarworks.uno.edu/td/1436/. Retrieved 4 November 2024.
- ↑ 14.0 14.1 14.2 14.3 "Dopamine-releasing agents". Dopamine Transporters: Chemistry, Biology and Pharmacology. Hoboken [NJ]: Wiley. July 2008. pp. 305–320. ISBN 978-0-470-11790-3. OCLC 181862653. https://bitnest.netfirms.com/external/Books/Dopamine-releasing-agents_c11.pdf.
- ↑ 15.0 15.1 15.2 "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse 39 (1): 32–41. January 2001. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ↑ "Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products". Neuropsychopharmacology 38 (4): 552–562. March 2013. doi:10.1038/npp.2012.204. PMID 23072836.
- ↑ 17.0 17.1 17.2 "Profiling CNS Stimulants with a High-Throughput Assay for Biogenic Amine Transporter Substractes". Problems of Drug Dependence 1999: Proceedings of the 61st Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc. NIDA Res Monogr. 180. 1999. pp. 1–476 (252). https://archives.nida.nih.gov/sites/default/files/180.pdf#page=261. "RESULTS. Methamphetamine and amphetamine potently released NE (IC50s = 14.3 and 7.0 nM) and DA (IC50s = 40.4 nM and 24.8 nM), and were much less potent releasers of 5-HT (IC50s = 740 nM and 1765 nM). Phentermine released all three biogenic amines with an order of potency NE (IC50 = 28.8 nM)> DA (IC50 = 262 nM)> 5-HT (IC50 = 2575 nM). Aminorex released NE (IC50 = 26.4 nM), DA (IC50 = 44.8 nM) and 5-HT (IC50 = 193 nM). Chlorphentermine was a very potent 5-HT releaser (IC50 = 18.2 nM), a weaker DA releaser (IC50 = 935 nM) and inactive in the NE release assay. Chlorphentermine was a moderate potency inhibitor of [3H]NE uptake (Ki = 451 nM). Diethylpropion, which is self-administered, was a weak DA uptake inhibitor (Ki = 15 µM) and NE uptake inhibitor (Ki = 18.1 µM) and essentially inactive in the other assays. Phendimetrazine, which is self-administered, was a weak DA uptake inhibitor (IC50 = 19 µM), a weak NE uptake inhibitor (8.3 µM) and essentially inactive in the other assays."
- ↑ "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology 37 (5): 1192–1203. April 2012. doi:10.1038/npp.2011.304. PMID 22169943.
- ↑ 19.0 19.1 19.2 "Characterization of a novel and potentially lethal designer drug (±)-cis-para-methyl-4-methylaminorex (4,4'-DMAR, or 'Serotoni')". Drug Testing and Analysis 6 (7–8): 684–695. 2014. doi:10.1002/dta.1668. PMID 24841869.
- ↑ 20.0 20.1 20.2 20.3 "Synthesis, characterization, and monoamine transporter activity of the new psychoactive substance 3',4'-methylenedioxy-4-methylaminorex (MDMAR)". Drug Testing and Analysis 7 (7): 555–564. July 2015. doi:10.1002/dta.1732. PMID 25331619.
- ↑ "Monoamine transporters and psychostimulant drugs". European Journal of Pharmacology 479 (1–3): 23–40. October 2003. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ↑ "Neurochemical mechanisms of phentermine and fenfluramine: Therapeutic and adverse effects". Drug Development Research 51 (2): 52–65. 2000. doi:10.1002/1098-2299(200010)51:2<52::AID-DDR2>3.0.CO;2-H. ISSN 0272-4391.
- ↑ "PDSP Database" (in zu). https://pdsp.unc.edu/databases/pdsp.php?receptorDD=&receptor=&speciesDD=&species=&sourcesDD=&source=&hotLigandDD=&hotLigand=&testLigandDD=&testFreeRadio=testFreeRadio&testLigand=aminorex&referenceDD=&reference=&KiGreater=&KiLess=&kiAllRadio=all&doQuery=Submit+Query.
- ↑ "BindingDB BDBM85705 Aminorex::CAS_2207-50-3::NSC_16630". Synapse (New York, N.Y.) 39 (1): 32–41. 2001. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707. https://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=85705. Retrieved 10 January 2025.
- ↑ 25.0 25.1 25.2 "The psychostimulant (±)-cis-4,4'-dimethylaminorex (4,4'-DMAR) interacts with human plasmalemmal and vesicular monoamine transporters". Neuropharmacology 138: 282–291. August 2018. doi:10.1016/j.neuropharm.2018.06.018. PMID 29908239. "Receptor-binding experiments suggest that 4,4'-DMAR exhibits no – or if at all only poor-affinity towards mouse and rat TAAR1. On the contrary, sub- (rat) and low-micromolar (mouse) affinities towards TAAR1 have been reported for MDMA (Simmler et al., 2013). The exact role of TAAR1 in amphetamine action remains far from being completely understood (Sitte and Freissmuth, 2015). However, TAAR1 appears to exert auto-inhibitory effects on monoaminergic neurons, thus regulates the release of the corresponding monoamines (Revel et al., 2011, 2012). TAAR1 is activated by a subset of amphetamines (Simmler et al., 2016). This observation has been linked to auto-inhibitory and neuroprotective effects of TAAR1 in amphetamine action (Miner et al., 2017; Revel et al., 2012; DiCara et al., 2011; Lindemann et al., 2008). The lack of agonist activity at TAAR1 might further contribute to long-term toxicity of 4,4'-DMAR, thus representing an interesting field for future investigations.".
- ↑ 26.0 26.1 26.2 26.3 "Pharmacological characterization of the aminorex analogs 4-MAR, 4,4'-DMAR, and 3,4-DMAR". Neurotoxicology 72: 95–100. May 2019. doi:10.1016/j.neuro.2019.02.011. PMID 30776375. Bibcode: 2019NeuTx..72...95R. "The methylated aminorex derivatives investigated in the present study did not interacted with TAAR1 receptors in contrast to amphetamine, MDMA, and several other phenethylamine derivatives (Revel et al., 2012; Simmler et al., 2016). Other aminorex-like ring-substituted 2- aminooxazolines have been shown to interact with TAAR1 receptors (Galley et al., 2016). However, they did not contain a 4-methyl group in contrast to the currently investigated compounds. Activity at TAAR1 may have auto-inhibitory effects on the monoaminergic action of amphetamine-type substances (Di Cara et al., 2011; Simmler et al., 2016). Therefore, the presently investigated compounds that did not bind to TAAR1 may exhibit greater stimulant properties compared to other amphetamines that also bind to TAAR1.".
- ↑ "In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1". J Pharmacol Exp Ther 357 (1): 134–144. April 2016. doi:10.1124/jpet.115.229765. PMID 26791601. https://d1wqtxts1xzle7.cloudfront.net/74120533/eae6c6e62565b82d46b4d111bbea0f77b9c2-libre.pdf?1635931703=&response-content-disposition=inline%3B+filename%3DIn_Vitro_Characterization_of_Psychoactiv.pdf&Expires=1746838268&Signature=Sy4fJ90yUhxs68314NxYsW5PAaNrBGePRu35WRR4PIF-3YC7Z~sLdnCn5wfqqbLg9bDEGdt~oW55ugMP3D3jgA0BoRI~~GOb0NQOwrtfUEQK1PQs1uuN9qg5Y1ct8z5NsABm44RgtukkwRMdU6fO7OlfIsQ68hOiFk129Ll7UYqldxD2f1xhE2fTTfsxSpb8cMCJzHn7-ItqLdwnAUPFK7WggDIjmY1kCnaHLwIxMwdJCAq8L6DYzSTg7pZkbR8qlou~GXbTPQt~gYpyZTJp5hgW-7V6K5wLlQ7Z2xE7B0f9wEfuc1W1QNafg125Tr-vvAe4LEGKXV58bnn1bpfWKw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA.
- ↑ "Trace Amines and Their Receptors". Pharmacol Rev 70 (3): 549–620. July 2018. doi:10.1124/pr.117.015305. PMID 29941461.
- ↑ "2-Amino-5-aryl-2-oxazolines. Potent New Anorectic Agents.". Journal of Medicinal Chemistry 6 (3): 266–272. May 1963. doi:10.1021/jm00339a011. PMID 14185981.
- ↑ "4,5-Disubstituted-1,3-oxazolidin-2-imine derivatives: a new class of orally bioavailable nitric oxide synthase inhibitor". Bioorganic & Medicinal Chemistry Letters 14 (2): 313–316. January 2004. doi:10.1016/j.bmcl.2003.11.010. PMID 14698148.
- ↑ "2-Amino-5-phenyl-2-oxazoline preparation" DE patent 2101424
- ↑ Albert MG, Ireland PG, "2-amino-5, 6-dihydro-4ii-1, 3-oxazines and a process for their preparation", US patent 3115494, issued 2 December 1963
- ↑ "Pharmacological therapy of obesity: past, present, and future". The Journal of Clinical Endocrinology and Metabolism 88 (6): 2462–2469. June 2003. doi:10.1210/jc.2003-030151. PMID 12788841.
Template:Chemical classes of psychoactive drugs
 |
