Chemistry:Methiopropamine
Methiopropamine (MPA), also known as N-methylthiopropamine, is an organic compound structurally related to methamphetamine.[1] Originally reported in 1942, the molecule consists of a thiophene group with an alkyl amine substituent at the 2-position.[1][2] It appeared for public sale in the United Kingdom in December 2010 as a "research chemical" or "legal high", recently branded as Blow.[3] It has limited popularity as a recreational stimulant.[1][4]
Pharmacology
Methiopropamine functions as a norepinephrine–dopamine reuptake inhibitor (NDRI) that is approximately 1.85 times more selective for norepinephrine than dopamine. It is approximately one-third as potent as dextroamphetamine as a norepinephrine reuptake inhibitor and one-fifth as much as a dopamine reuptake inhibitor. It displays negligible activity as a serotonin reuptake inhibitor.[5][6]
Methiopropamine has the potential for significant acute toxicity with cardiovascular, gastrointestinal, and psychotic symptoms.[7]
Metabolism
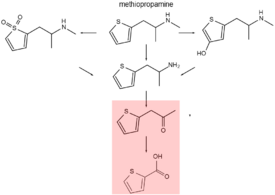
For N-alkyl amphetamines, deamination and N-dealkylation are the major elimination pathways and renal excretion is a minor one.[8]
Methiopropamine is metabolized into active thiopropamine, 4-hydroxymethiopropamine and thiophene S-oxides.[9][10] These N-demethylated metabolites are further deaminated by the cytochrome P450 enzyme CYP2C19 in the liver transforming them into inactive 1-(thiophen-2-yl)-2-propan-2-one which can be seen as a phenylacetone derivative.[11][12]
Thiophene-2-carboxylic acid is the final major metabolic product. It is very hydrophilic and is excreted in urine. Methiopropamine and especially thiopropamine are also excreted renally, unchanged.
Synthesis
There is a four-step synthesis of methiopropamine. It begins with (thiophen-2-yl)magnesium bromide, which is reacted with propylene oxide, yielding 1-(thiophen-2-yl)-2-hydroxypropane which is reacted with phosphorus tribromide, yielding 1-(thiophen-2-yl)-2-bromopropane which is finally reacted with methylamine, yielding 1-(thiophen-2-yl)-2-methylaminopropane.[13] Methiopropamine is off-white, yellowish powder.[14]

Legal status
China
As of October 2015 MPA is a controlled substance in China.[15]
Finland
Methiopropamine is illegal in Finland, it is scheduled in "government decree on narcotic substances, preparations and plants".[16]
Germany
United Kingdom
Following the ban on ethylphenidate, authorities noticed an increase in methiopropamine use by injecting users. The ACMD suggested it be banned on 18 November 2015[17] as it had similar effects to ethylphenidate. The government enacted a temporary drug control order a week later which came into force on 27 November 2015.[18] Though ordinarily the TCDO would only last 1 year, the ACMD reported that since its invocation prevalence of MPA had significantly decreased, and that it had been challenging to collect information about the drug. As a result of this, they requested that the TCDO be extended a further year.[19]
Methiopropanine was made a Class B controlled drug under the Misuse of Drugs Act 1971 (as amended) (Amendment)(No.2) Order 2017 [SI 2017/1114], this came into effect on the 27th of November 2017.
United States
Methiopropamine is scheduled at the federal level in the United States.[20] The DEA had planned to place methiopropamine in Schedule I of Controlled Substances and was accepting public comments until October 4, 2021. Later, the compound was placed in Schedule I.[21]
Florida
Methiopropamine is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[22]
Tasmania (Australia)
See also
- 5-MMPA
- α-Pyrrolidinopentiothiophenone (α-PVT)
- Thiopropamine, demethylated counterpart
- Propylhexedrine, another ring substituted stimulant used as over-the-counter decongestant
- Thiothinone
References
- ↑ 1.0 1.1 1.2 "Understanding methiopropamine, a new psychoactive substance: an in-depth review on its chemistry, pharmacology and implications to human health". Int J Legal Med 138 (4): 1295–1306. July 2024. doi:10.1007/s00414-024-03201-7. PMID 38424369.
- ↑ "α-Thienylaminoalkanes". Journal of the American Chemical Society 64 (3): 477–80. March 1942. doi:10.1021/ja01255a001. Bibcode: 1942JAChS..64..477B.
- ↑ "The syntheses of 1-(2-thienyl)-2-(methylamino) propane (methiopropamine) and its 3-thienyl isomer for use as reference standards". Drug Testing and Analysis 5 (3): 145–9. March 2013. doi:10.1002/dta.298. PMID 21770051.
- ↑ "Methiopropamine Thread". UKChemicalResearch.org. https://www.ukchemicalresearch.org/Thread-MPA.
- ↑ "Neurochemical profiles of some novel psychoactive substances". European Journal of Pharmacology 700 (1–3): 147–51. January 2013. doi:10.1016/j.ejphar.2012.12.006. PMID 23261499.
- ↑ "The expression of methiopropamine-induced locomotor sensitization requires dopamine D2, but not D1, receptor activation in the rat". Behavioural Brain Research 311: 403–407. September 2016. doi:10.1016/j.bbr.2016.05.060. PMID 27265782.
- ↑ "Acute toxicity associated with analytically confirmed recreational use of methiopropamine (1-(thiophen-2-yl)-2-methylaminopropane)". Journal of Medical Toxicology 10 (3): 299–302. September 2014. doi:10.1007/s13181-014-0399-y. PMID 24706157.
- ↑ "Deuterium isotope effects in the metabolism of N-alkylsubstituted amphetamines in man". Clinica Chimica Acta; International Journal of Clinical Chemistry 34 (2): 333–44. September 1971. doi:10.1016/0009-8981(71)90187-2. PMID 5113570.
- ↑ "Chemical and Biological Oxidation of Thiophene: Preparation and Complete Characterization of Thiophene S-Oxide Dimers and Evidence for Thiophene S-Oxide as an Intermediate in Thiophene Metabolism in Vivo and in Vitro". Journal of the American Chemical Society 119 (7): 1565–71. 1997. doi:10.1021/ja962466g. Bibcode: 1997JAChS.119.1565T.
- ↑ "Evidence for thiophene-S-oxide as a primary reactive metabolite of thiophene in vivo: formation of a dihydrothiophene sulfoxide mercapturic acid". Biochemical and Biophysical Research Communications 186 (3): 1624–30. August 1992. doi:10.1016/S0006-291X(05)81594-3. PMID 1510686. Bibcode: 1992BBRC..186.1624D.
- ↑ "Deamination of amphetamines by cytochromes P450: studies on substrate specificity and regioselectivity with microsomes and purified CYP2C subfamily isozymes". The Journal of Toxicological Sciences 22 (1): 65–73. February 1997. doi:10.2131/jts.22.65. PMID 9076658.
- ↑ "2-methiopropamine, a thiophene analogue of methamphetamine: studies on its metabolism and detectability in the rat and human using GC-MS and LC-(HR)-MS techniques". Analytical and Bioanalytical Chemistry 405 (10): 3125–35. April 2013. doi:10.1007/s00216-013-6741-4. PMID 23361230. https://boris.unibe.ch/44923/.
- ↑ "Methiopropamine: An Analytical Profile". Microgram Journal 8 (2): 53–57. 2011. https://www.justice.gov/dea/pr/microgram-journals/2011/mj8-2_53-57.pdf.
- ↑ https://www.erowid.org/chemicals/show_image.php?i=methiopropamine/methiopropamine_powder__i2011e0003_disp.jpg
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ finlex.fi
- ↑ Advisory Council on the Misuse of Drugs (25 November 2015). "Methiopropamine (MPA): A review of the evidence of use and harm". UK Home Office. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/479996/23_November_-_TCDO_Report_for_MPA.pdf.
- ↑ "The Misuse of Drugs Act 1971 (Temporary Class Drug) (No. 3) Order 2015". UK Government. 23 November 2015. http://www.legislation.gov.uk/uksi/2015/1929/made.
- ↑ "Re: Temporary Class Drug Order on methiopropamine". 2016. https://www.gov.uk/government/publications/temporary-class-drug-order-on-methiopropamine.
- ↑ "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I.". http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm.
- ↑ "Federal Register". National Archives. December 9, 2022. https://www.federalregister.gov/agencies/drug-enforcement-administration.
- ↑ Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL
External links
- Methiopropamine at erowid.org
- Methiopropamine at isomerdesign.com
- Methiopropamine at psychonautwiki.org
 |
