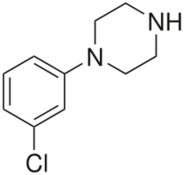
Chemistry:Meta-Chlorophenylpiperazine
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, intranasal, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver (CYP2D6)[1] |
| Elimination half-life | 4–14 hours[1][2] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C10H13ClN2 |
| Molar mass | 196.68 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
meta-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s.[3][4] It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as "ecstasy" in Europe and the United States.[5][6]
Despite its advertisement as a recreational substance, mCPP is actually generally considered to be an unpleasant experience and is not desired by drug users.[5] It lacks any reinforcing effects, but has "psychostimulant, anxiety-provoking, and hallucinogenic effects."[7][8][9][10] It is also known to produce dysphoric, depressive, and anxiogenic effects in rodents and humans,[11][12] and can induce panic attacks in individuals susceptible to them.[13][14][15][16] It also worsens obsessive–compulsive symptoms in people with the disorder.[17][18][19]
mCPP is known to induce headaches in humans and has been used for testing potential antimigraine medications.[20][21][22] It has potent anorectic effects and has encouraged the development of selective 5-HT2C receptor agonists for the treatment of obesity as well.[23][24][25][26]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Refs |
|---|---|---|---|
| NET | 1,940–4,360 | Human | [27] |
| DAT | ND | ND | ND |
| 5-HT1A | 44–400 | Human | [28][29] |
| 5-HT1B | 89–501 | Human | [28][30] |
| 5-HT1D | 210–1,300 | Human | [29][31] |
| 5-HT1E | ND | ND | ND |
| 5-HT1F | ND | ND | ND |
| 5-HT2A | 32–398 | Human | [28][10][29][32] |
| 5-HT2B | 3.2–63 | Human | [28][33][34] |
| 5-HT2C | 3.4–251 | Human | [28][10][32][35] |
| 5-HT3 | 427 | Human | [28] |
| 5-HT4 | ND | ND | ND |
| 5-HT5A | 1,354 | Human | [28] |
| 5-HT6 | 1,748 | Human | [28] |
| 5-HT7 | 163 | Human | [28] |
| α1 | 97–2,900 | Human | [27][29] |
| α1A | 1,386 | Human | [28] |
| α1B | 915 | Human | [28] |
| α1D | ND | ND | ND |
| α2 | 112–570 | Human | [27][29] |
| α2A | 145 | Human | [28] |
| α2B | 106 | Human | [28] |
| α2C | 124 | Human | [28] |
| β | 2,500 | Human | [29] |
| β1 | 2,359 | Human | [28] |
| β2 | 3,474 | Human | [28] |
| D1 | 7,000 | Human | [29] |
| D2 | >10,000 | Human | [29] |
| D3 | >10,000 | Rat | [28] |
| D4 | ND | ND | ND |
| D5 | >10,000 | Human | [28] |
| H1 | 326 | Human | [28] |
| mAChRs | >10,000 | Human | [29] |
| nAChRs | >10,000 | Human | [28] |
| σ1 | ND | ND | ND |
| σ2 | 8,350 | Rat | [28] |
| I1 | 759 | Rat | [28] |
| VDCC | 6,043 | Rat | [28] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||
mCPP possesses significant affinity for the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, and 5-HT7 receptors, as well as the SERT.[28][36] It also has some affinity for α1-adrenergic, α2-adrenergic, H1, I1, and NET.[28][37] It behaves as an agonist at most serotonin receptors.[38][39] mCPP has been shown to act not only as a serotonin reuptake inhibitor but as a serotonin releasing agent as well.[40]
mCPP's strongest actions are at the 5-HT2B and 5-HT2C receptors and its discriminative cue is mediated primarily by 5-HT2C.[28][41][42] Its negative effects such as anxiety, headaches, and appetite loss are likely mediated by its actions on the 5-HT2C receptor.[12][23][43] Other effects of mCPP include nausea, hypoactivity, and penile erection, the latter two the result of increased 5-HT2C activity and the former likely via 5-HT3 stimulation.[44][45][46] mCPP is known to induce migraines and this may be mediated by serotonin 5-HT2B receptor agonism.[47]
In comparison studies, mCPP has approximately 10-fold selectivity for the human 5-HT2C receptor over the human 5-HT2A and 5-HT2B receptors (Ki = 3.4 nM vs. 32.1 and 28.8 nM).[10] It acts as a partial agonist of the human 5-HT2A[48] and 5-HT2C[49] receptors but as an antagonist of the human 5-HT2B receptors.[50]
mCPP produces the head-twitch response, a behavioral proxy of psychedelic effects, in rodents, and hence may be hallucinogenic in humans.[51] Despite this however, mCPP has been described as non-hallucinogenic in humans.[52] In any case, hallucinations have occasionally been reported when large doses of mCPP are taken.[52]
Pharmacokinetics
mCPP is metabolized via the CYP2D6 isoenzyme by hydroxylation to para-hydroxy-mCPP (p-OH-mCPP) and this plays a major role in its metabolism.[1][53][54] The elimination half-life of mCPP is 4 to 14 hours.[1]
mCPP is a metabolite of a variety of other piperazine drugs including trazodone, nefazodone, etoperidone, mepiprazole, cloperidone, peraclopone, and BRL-15,572.[54][additional citation(s) needed] It is formed by dealkylation via CYP3A4.[54] Caution should be exercised in concomitant administration of CYP2D6 inhibitors such as bupropion, fluoxetine, paroxetine, and thioridazine with drugs that produce mCPP as a metabolite as these drugs are known to increase concentrations of the parent molecule (e.g., trazodone) and of mCPP.[53][1]
Chemistry
Analogues
Analogues of mCPP include:
- 1-Benzylpiperazine (BZP)
- 1-Methyl-4-benzylpiperazine (MBZP)
- 1,4-Dibenzylpiperazine (DBZP)
- 3-Trifluoromethylphenylpiperazine (TFMPP)
- 3,4-Methylenedioxy-1-benzylpiperazine (MDBZP)
- 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP)
- 4-Fluorophenylpiperazine (pFPP)
- 4-Methoxyphenylpiperazine (MeOPP)
Some additional analogues include quipazine, ORG-12962, and 3C-PEP.
Society and culture


Legal status
Belgium
mCPP is illegal in Belgium.[55]
Brazil
mCPP is illegal in Brazil.[56]
Canada
mCPP is not a controlled drug in Canada.
China
As of October 2015 mCPP is a controlled substance in China.[57]
Czech Republic
mCPP is legal in the Czech Republic.[58]
Denmark
mCPP is illegal in Denmark.[59]
Finland
mCPP is illegal in Finland.
Germany
mCPP is illegal in Germany.
Hungary
mCPP is illegal in Hungary since 2012.
Japan
mCPP is illegal in Japan since 2006.
Netherlands
mCPP is legal in the Netherlands.[60]
New Zealand
Based on the recommendation of the EACD, the New Zealand government has passed legislation which placed BZP, along with the other piperazine derivatives TFMPP, mCPP, pFPP, MeOPP and MBZP, into Class C of the New Zealand Misuse of Drugs Act 1975. A ban was intended to come into effect in New Zealand on December 18, 2007, but the law change did not go through until the following year, and the sale of BZP and the other listed piperazines became illegal in New Zealand as of 1 April 2008. An amnesty for possession and usage of these drugs remained until October 2008, at which point they became completely illegal.[61] However, mCPP is legally used for scientific research.
Norway
mCPP is illegal in Norway.
Russia
mCPP is illegal in Russia.
Sweden
mCPP is illegal in Sweden.
Poland
mCPP is illegal in Poland.
United States
mCPP is not scheduled at the federal level in the United States,[62] but it is possible that it could be considered a controlled substance analog of BZP, in which case purchase, sale, or possession could be prosecuted under the Federal Analog Act.
However, "chlorophenylpiperazine" is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in this state.[63]
United Arab Emirates
mCPP is illegal in the UAE, the country has strict drug laws, and many psychoactive substances are classified as controlled or prohibited.[64]
Turkey
mCPP is illegal in Turkey since 20/05/2009.[65]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Metabolism of some "second"- and "fourth"-generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine". Cellular and Molecular Neurobiology 19 (4): 427–442. August 1999. doi:10.1023/a:1006953923305. PMID 10379419.
- ↑ The American Psychiatric Association Publishing Textbook of Psychopharmacology, Fifth Edition. American Psychiatric Pub. 2017. pp. 460–. ISBN 978-1-58562-523-9. https://books.google.com/books?id=KfHEDgAAQBAJ&pg=PA460.
- ↑ "Methylone and mCPP, two new drugs of abuse?". Addiction Biology 10 (4): 321–323. December 2005. doi:10.1080/13556210500350794. PMID 16318952. http://www3.interscience.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=1355-6215&date=2005&volume=10&issue=4&spage=321.
- ↑ "[Metachlorophenylpiperazine (mCPP): a new designer drug]" (in fr). Therapie 61 (6): 523–530. 2006. doi:10.2515/therapie:2006093. PMID 17348609.
- ↑ 5.0 5.1 "mCPP: an undesired addition to the ecstasy market". Journal of Psychopharmacology 24 (9): 1395–1401. September 2010. doi:10.1177/0269881109102541. PMID 19304863.
- ↑ "Content of ecstasy in the Netherlands: 1993-2008". Addiction 104 (12): 2057–2066. December 2009. doi:10.1111/j.1360-0443.2009.02707.x. PMID 19804461.
- ↑ World Health Organization. "1-(3-chlorophenyl) piperazine (mCPP) - Expert peer review on pre-review report". http://158.232.12.119/entity/medicines/areas/quality_safety/5.3cmCPPpre-review.pdf.
- ↑ "The subjective effects of MDMA and mCPP in moderate MDMA users". Drug and Alcohol Dependence 65 (1): 97–101. December 2001. doi:10.1016/s0376-8716(01)00146-6. PMID 11714594.
- ↑ "Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with d-amphetamine and mCPP". Drug and Alcohol Dependence 72 (1): 33–44. October 2003. doi:10.1016/S0376-8716(03)00172-8. PMID 14563541.
- ↑ 10.0 10.1 10.2 10.3 "Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors". Naunyn-Schmiedeberg's Archives of Pharmacology 359 (1): 1–6. January 1999. doi:10.1007/pl00005315. PMID 9933142.
- ↑ "1-(m-Chlorophenyl)piperazine induces depressogenic-like behaviour in rodents by stimulating the neuronal 5-HT(2A) receptors: proposal of a modified rodent antidepressant assay". European Journal of Pharmacology 608 (1–3): 32–41. April 2009. doi:10.1016/j.ejphar.2009.02.041. PMID 19269287.
- ↑ 12.0 12.1 "Anxiogenic-like effects of mCPP and TFMPP in animal models are opposed by 5-HT1C receptor antagonists". European Journal of Pharmacology 164 (3): 445–454. May 1989. doi:10.1016/0014-2999(89)90252-5. PMID 2767117.
- ↑ "Anxiogenic effects of m-CPP in patients with panic disorder: comparison to caffeine's anxiogenic effects". Biological Psychiatry 30 (10): 973–984. November 1991. doi:10.1016/0006-3223(91)90119-7. PMID 1756202. https://zenodo.org/record/1253832.
- ↑ "Serotonin function in anxiety. II. Effects of the serotonin agonist MCPP in panic disorder patients and healthy subjects". Psychopharmacology 92 (1): 14–24. 1987. doi:10.1007/bf00215473. PMID 3110824.
- ↑ "Behavioural effects of rapid intravenous administration of meta-chlorophenylpiperazine (m-CPP) in patients with generalized social anxiety disorder, panic disorder and healthy controls". European Neuropsychopharmacology 17 (10): 637–642. October 2007. doi:10.1016/j.euroneuro.2007.03.005. PMID 17481859.
- ↑ "Behavioural effects of rapid intravenous administration of meta-chlorophenylpiperazine in patients with panic disorder and controls". European Neuropsychopharmacology 14 (5): 413–417. October 2004. doi:10.1016/j.euroneuro.2004.01.001. PMID 15336303.
- ↑ "Serotonergic function in obsessive-compulsive disorder. Behavioral and neuroendocrine responses to oral m-chlorophenylpiperazine and fenfluramine in patients and healthy volunteers". Archives of General Psychiatry 49 (1): 21–28. January 1992. doi:10.1001/archpsyc.1992.01820010021003. PMID 1728249.
- ↑ "Acute intravenous administration of ondansetron and m-CPP, alone and in combination, in patients with obsessive-compulsive disorder (OCD): behavioral and biological results". Psychiatry Research 79 (1): 11–20. June 1998. doi:10.1016/S0165-1781(98)00029-8. PMID 9676822. https://zenodo.org/record/1259861.
- ↑ "Metergoline blocks the behavioral and neuroendocrine effects of orally administered m-chlorophenylpiperazine in patients with obsessive-compulsive disorder". Biological Psychiatry 29 (5): 418–426. March 1991. doi:10.1016/0006-3223(91)90264-M. PMID 2018816.
- ↑ "The serotonergic agent m-chlorophenylpiperazine induces migraine attacks: A controlled study". Neurology 55 (1): 136–139. July 2000. doi:10.1212/wnl.55.1.136. PMID 10891925.
- ↑ "Investigations into migraine pathogenesis: time course for effects of m-CPP, BW723C86 or glyceryl trinitrate on appearance of Fos-like immunoreactivity in rat trigeminal nucleus caudalis (TNC)". Cephalalgia 21 (1): 46–52. February 2001. doi:10.1046/j.1468-2982.2001.00157.x. PMID 11298663.
- ↑ "Anxiolytic effects of dotarizine, a possible antimigraine drug". Methods and Findings in Experimental and Clinical Pharmacology 17 (10): 659–668. December 1995. PMID 9053586.
- ↑ 23.0 23.1 "Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors". Psychopharmacology 96 (1): 93–100. 1988. doi:10.1007/BF02431539. PMID 2906446.
- ↑ "5-HT2C receptor activation decreases appetite and body weight in obese subjects". Psychopharmacology 133 (3): 309–312. October 1997. doi:10.1007/s002130050407. PMID 9361339. http://link.springer.de/link/service/journals/00213/bibs/7133003/71330309.htm.
- ↑ "Agonist diversity in 5-HT(2C) receptor-mediated weight control in rats". Psychopharmacology 178 (2–3): 241–249. March 2005. doi:10.1007/s00213-004-2019-z. PMID 15719229.
- ↑ "Serotonergic drugs : effects on appetite expression and use for the treatment of obesity". Drugs 67 (1): 27–55. 2007. doi:10.2165/00003495-200767010-00004. PMID 17209663.
- ↑ 27.0 27.1 27.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9400006 - ↑ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 28.10 28.11 28.12 28.13 28.14 28.15 28.16 28.17 28.18 28.19 28.20 28.21 28.22 28.23 28.24 28.25 Cite error: Invalid
<ref>tag; no text was provided for refs namedPDSP - ↑ 29.0 29.1 29.2 29.3 29.4 29.5 29.6 29.7 29.8 "1-(m-chlorophenyl)piperazine (mCPP) interactions with neurotransmitter receptors in the human brain". Biological Psychiatry 25 (5): 569–575. March 1989. doi:10.1016/0006-3223(89)90217-5. PMID 2537663.
- ↑ "Molecular biology of 5-HT receptors". Neuropharmacology 33 (3–4): 275–317. 1994. doi:10.1016/0028-3908(94)90059-0. PMID 7984267.
- ↑ "Primary structure and functional characterization of a human 5-HT1D-type serotonin receptor". Molecular Pharmacology 40 (2): 143–148. August 1991. doi:10.1016/S0026-895X(25)12918-0. PMID 1652050.
- ↑ 32.0 32.1 "The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors". British Journal of Pharmacology 115 (4): 622–628. June 1995. doi:10.1111/j.1476-5381.1995.tb14977.x. PMID 7582481.
- ↑ "Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications". Circulation 102 (23): 2836–2841. December 2000. doi:10.1161/01.cir.102.23.2836. PMID 11104741.
- ↑ "Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells". British Journal of Pharmacology 128 (1): 13–20. September 1999. doi:10.1038/sj.bjp.0702751. PMID 10498829.
- ↑ "Indoline derivatives as 5-HT(2C) receptor agonists". Bioorganic & Medicinal Chemistry Letters 14 (9): 2367–2370. May 2004. doi:10.1016/j.bmcl.2003.05.001. PMID 15081042.
- ↑ "Central serotonin receptors as targets for drug research". J Med Chem 30 (1): 1–12. January 1987. doi:10.1021/jm00384a001. PMID 3543362. "Table II. Affinities of Selected Phenalkylamines for 5-HT1 and 5-HT2 Binding Sites".
- ↑ "The effects of administration of mCPP on psychological, cognitive, cardiovascular, hormonal and MHPG measurements in human volunteers". International Clinical Psychopharmacology 9 (3): 173–178. September 1994. doi:10.1097/00004850-199409000-00005. PMID 7814826.
- ↑ "Chlorophenylpiperazine: a central serotonin agonist causing powerful anorexia in rats". Naunyn-Schmiedeberg's Archives of Pharmacology 308 (2): 159–163. August 1979. doi:10.1007/BF00499059. PMID 503247.
- ↑ "Trazodone and its active metabolite m-chlorophenylpiperazine as partial agonists at 5-HT1A receptors assessed by [35S]GTPgammaS binding". Journal of Psychopharmacology 19 (3): 235–241. May 2005. doi:10.1177/0269881105051526. PMID 15888508.
- ↑ "Serotonin-releasing effects of substituted piperazines in vitro". Biochemical Pharmacology 33 (9): 1531–1535. May 1984. doi:10.1016/0006-2952(84)90424-6. PMID 6610423.
- ↑ "Involvement of 5-HT2C receptors in mediating the discriminative stimulus properties of m-chlorophenylpiperazine (mCPP)". European Journal of Pharmacology 257 (1–2): 27–38. May 1994. doi:10.1016/0014-2999(94)90690-4. PMID 8082704.
- ↑ "Discriminative stimulus properties of mCPP: evidence for a 5-HT2C receptor mode of action". Psychopharmacology 137 (3): 292–302. June 1998. doi:10.1007/s002130050622. PMID 9683007. http://link.springer.de/link/service/journals/00213/bibs/8137003/81370292.htm.
- ↑ "Evidence that mCPP may have behavioural effects mediated by central 5-HT1C receptors". British Journal of Pharmacology 94 (1): 137–147. May 1988. doi:10.1111/j.1476-5381.1988.tb11508.x. PMID 3401632.
- ↑ "Exploratory hypoactivity induced by m-trifluoromethylphenylpiperazine (TFMPP) and m-chlorophenylpiperazine (m-CPP)". Journal of Neural Transmission. Parkinson's Disease and Dementia Section 1 (3): 207–218. 1989. doi:10.1007/BF02248670. PMID 2775468.
- ↑ "m-Chlorophenylpiperazine decreases food intake in a test meal". Psychopharmacology 116 (1): 120–122. September 1994. doi:10.1007/BF02244883. PMID 7862925.
- ↑ "Penile erection and yawning induced by 5-HT1C receptor agonists in male rats: relationship with dopaminergic and oxytocinergic transmission". European Journal of Pharmacology 261 (1–2): 149–155. August 1994. doi:10.1016/0014-2999(94)90313-1. PMID 8001637.
- ↑ "Putative role of 5-HT2B receptors in migraine pathophysiology". Cephalalgia 37 (4): 365–371. April 2017. doi:10.1177/0333102416646760. PMID 27127104. https://nbn-resolving.org/urn:nbn:de:bvb:29-opus4-112198.
- ↑ "m-chlorophenylpiperazine and m-trifluoromethylphenylpiperazine are partial agonists at cloned 5-HT2A receptors expressed in fibroblasts". The Journal of Pharmacology and Experimental Therapeutics 271 (2): 1122–1126. November 1994. doi:10.1016/S0022-3565(25)23805-8. PMID 7965773.
- ↑ "Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells". British Journal of Pharmacology 128 (1): 13–20. September 1999. doi:10.1038/sj.bjp.0702751. PMID 10498829.
- ↑ "m-Chlorophenylpiperazine (mCPP) is an antagonist at the cloned human 5-HT2B receptor". NeuroReport 7 (9): 1457–1460. June 1996. doi:10.1097/00001756-199606170-00002. PMID 8856697.
- ↑ "Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists". Pharmacol Biochem Behav 69 (3–4): 643–652. 2001. doi:10.1016/s0091-3057(01)00552-4. PMID 11509227.
- ↑ 52.0 52.1 "Psychedelics: preclinical insights provide directions for future research". Neuropsychopharmacology 49 (1): 119–127. January 2024. doi:10.1038/s41386-023-01567-7. PMID 36932180.
- ↑ 53.0 53.1 "Human CYP2D6 and metabolism of m-chlorophenylpiperazine". Biological Psychiatry 44 (11): 1185–1191. December 1998. doi:10.1016/S0006-3223(97)00483-6. PMID 9836023.
- ↑ 54.0 54.1 54.2 "Current awareness of piperazines: pharmacology and toxicology". Drug Testing and Analysis 3 (7–8): 430–438. 2011. doi:10.1002/dta.307. PMID 21744514.
- ↑ "EMCDDA | News". http://www.emcdda.europa.eu//html.cfm//index5176EN.html?sLanguageISO=EN&nNodeID=5176&pluginMethod=eldd.shownewsdetails&id=20/12/2006BELGIUM:+New+substances+controlled+include+mCPP+and+khat.
- ↑ "ANVISA resolution - Portaria SVS/MS 344/98". http://www.anvisa.gov.br/divulga/noticias/2008/051108_2.htm.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Legislativa". http://www.mzcr.cz/Odbornik/obsah/legislativa_1051_3.html.
- ↑ "Erowid Psychoactive Vaults : Law : Countries : Denmark (DK) : Denmark bans 2C-T-4, TFMPP, BZP, mCPP, MeOPP". https://www.erowid.org/psychoactives/law/countries/denmark/denmark_law_info1.shtml.
- ↑ Koninkrijksrelaties, Ministerie van Binnenlandse Zaken en. "Opiumwet". https://wetten.overheid.nl/BWBR0001941/2017-05-25.
- ↑ "Misuse of Drugs (Classification of BZP) Amendment Bill 2008". http://www.parliament.nz/en-NZ/PB/Legislation/Bills/d/3/d/00DBHOH_BILL8220_1-Misuse-of-Drugs-Classification-of-BZP-Amendment.htm.
- ↑ "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I.". http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm.
- ↑ "Statutes & Constitution :View Statutes : Online Sunshine". http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html.
- ↑ "Drugs | The Official Portal of the UAE Government" (in en). https://u.ae/en/information-and-services/health-and-fitness/drugs-and-controlled-medicines/drugs.
- ↑ "Başbakanlık Mevzuatı Geliştirme ve Yayın Genel Müdürlüğü". https://www.resmigazete.gov.tr/eskiler/2009/05/20090520-3.htm.
External links
 |
