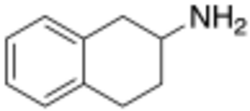
Chemistry:2-Aminotetralin
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C10H13N |
| Molar mass | 147.221 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
2-Aminotetralin (2-AT), also known as 1,2,3,4-tetrahydronaphthalen-2-amine (THN), is a stimulant drug with a chemical structure consisting of a tetralin group combined with an amine.[1][2]
2-AT is a rigid analogue of phenylisobutylamine and fully substitutes for d-amphetamine in rat discrimination tests, although at one eighth the potency.[1] It has been shown to inhibit the reuptake of serotonin and norepinephrine, and likely induces their release as well.[3][4] It is also likely to act on dopamine on account of its full substitution of d-amphetamine in rodent studies.[1]
Chemical derivatives
A number of derivatives of 2-aminotetralin exist, including:
See also
- 2-Aminodilin
- 2-Aminoindane
References
- ↑ 1.0 1.1 1.2 "Structural variation and (+)-amphetamine-like discriminative stimulus properties". Pharmacology, Biochemistry, and Behavior 38 (3): 581–6. March 1991. doi:10.1016/0091-3057(91)90017-V. PMID 2068194.
- ↑ "Actions of dexamphetamine and amphetamine-like amines in chickens with brain transections". British Journal of Pharmacology 42 (4): 522–42. August 1971. doi:10.1111/j.1476-5381.1971.tb07138.x. PMID 5116035.
- ↑ "Evidence for inhibition of the reuptake of 5-hydroxytryptamine and noradrenaline by tetrahydronaphthylamine in rat brain". British Journal of Pharmacology 42 (2): 281–6. June 1971. doi:10.1111/j.1476-5381.1971.tb07109.x. PMID 5091160.
- ↑ "Role of noradrenaline and 5-hydroxytryptamine in tetrahydronaphthylamine-induced temperature changes in the rat". British Journal of Pharmacology 43 (1): 1–9. September 1971. doi:10.1111/j.1476-5381.1971.tb07151.x. PMID 4257629.
 |

