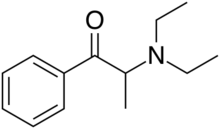
Chemistry:Amfepramone
 | |
| Clinical data | |
|---|---|
| Trade names | Tenuate, Tepanil, Nobesine, others |
| Other names | Diethylpropion, Diethylcathinone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682037 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 4–6 hours (metabolites)[6] |
| Excretion | Urine (>75%)[6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C13H19NO |
| Molar mass | 205.301 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Amfepramone, also known as diethylpropion, is a stimulant drug of the phenethylamine, amphetamine, and cathinone classes that is used as an appetite suppressant.[7][8] It is used in the short-term management of obesity, along with dietary and lifestyle changes.[7] Amfepramone has a similar chemical structure to the antidepressant and smoking cessation aid bupropion (previously called amfebutamone), which has also been developed as a weight-loss medicine when in a combination product with naltrexone.[9]
Pharmacology
Amfepramone itself lacks any affinity for the monoamine transporters and instead functions as a prodrug to ethcathinone.[10] Ethcathinone (and therefore amfepramone as well) is a very weak dopaminergic and serotonergic, and is approximately 10× and 20× stronger on norepinephrine in comparison, respectively.[10]
Chemistry
Amfepramone can be synthesized from propiophenone by bromination, followed by reaction with diethylamine.[11][12]
Society and culture
Names
Another medically utilized name is diethylpropion (British Approved Name (BAN) and Australian Approved Name (AAN)). Chemical names include: α-methyl-β-keto-N,N-diethylphenethylamine, N,N-diethyl-β-ketoamphetamine and N,N-diethylcathinone. Brand names include: Anorex, Linea, Nobesine, Prefamone, Regenon, Tepanil and Tenuate.
Legal status
Amfepramone is classified as a Schedule IV controlled substance in the United States. In the UK amfepramone is a class C drug [13] and as a medicine, it is a Schedule 3 Controlled Drug which requires safe custody.
As of June 2022, the safety committee of the European Medicines Agency (EMA) recommends the withdrawal of marketing authorizations for amfepramone.[14][5]
Recreational use
The authors of several studies of amfepramone claim that the substance has a relatively low potential for causing addiction in users.[15][16][3][17]
References
- ↑ "Tenuate Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=16693.
- ↑ "Nobesine Product information". 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=9263.
- ↑ 3.0 3.1 "Tepanil (diethylpropion hydrochloride) tablet, extended release". Dailymed. National Institutes of Health. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=79b15110-e484-4b84-8947-15653746773c.
- ↑ "Amfepramone. List of nationally authorized medicinal products". https://www.ema.europa.eu/en/documents/psusa/amfepramone-list-nationally-authorised-medicinal-products-psusa/00000138/202006_en.pdf.
- ↑ 5.0 5.1 "Amfepramone-containing medicinal products". 12 February 2021. https://www.ema.europa.eu/en/medicines/human/referrals/amfepramone-containing-medicinal-products.
- ↑ 6.0 6.1 "SPC-DOC_PL 16133-0001". Medicines Healthcare products Regulatory Agency. Essential Nutrition Ltd. 18 November 2011. http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1396847771265.pdf.[yes|permanent dead link|dead link}}]
- ↑ 7.0 7.1 "Diethylpropion Hydrochloride". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 30 January 2013. https://www.medicinescomplete.com/mc/martindale/current/1475-e.htm.
- ↑ "TGA Approved Terminology for Medicines, Section 1 – Chemical Substances". Therapeutic Goods Administration, Department of Health and Ageing, Australian Government. July 1999. p. 42. http://www.tga.gov.au/pdf/medicines-approved-terminology-chemical.pdf.
- ↑ "Pharmacological and neurotoxicological actions mediated by bupropion and diethylpropion". New Concepts of Psychostimulant Induced Neurotoxicity. International Review of Neurobiology. 88. 2009. pp. 223–55. doi:10.1016/S0074-7742(09)88009-4. ISBN 9780123745040.
- ↑ 10.0 10.1 "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry 6 (17): 1845–59. 2006. doi:10.2174/156802606778249766. PMID 17017961. https://zenodo.org/record/1235860. Retrieved 7 September 2020.
- ↑ Schutte J, "Anorexigenic Propiophenones", US patent 3001910, issued 1961-09-26, assigned to Temmler-Werke
- ↑ "Synthetic Homologs of d,l-Ephedrine". Journal of the American Chemical Society 50 (8): 2287–2292. 1928. doi:10.1021/ja01395a032.
- ↑ "Class C Drugs". Schedule 2 Controlled Drugs. UK Legislation. http://www.legislation.gov.uk/ukpga/1971/38/schedule/2.
- ↑ "EMA recommends withdrawal of marketing authorisation for amfepramone medicines". 10 June 2022. https://www.ema.europa.eu/en/news/ema-recommends-withdrawal-marketing-authorisation-amfepramone-medicines.
- ↑ "Diethylpropion (tenuate): an infrequently abused anorectic". Psychosomatics 18 (1): 28–33. 1977. doi:10.1016/S0033-3182(77)71101-6. PMID 850721.
- ↑ "Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse". Journal of Psychopharmacology 23 (4): 419–27. June 2009. doi:10.1177/0269881109103113. PMID 19329547.
- ↑ "Habituation to diethylpropion (Tenuate)". Canadian Medical Association Journal 88 (18): 943–4. May 1963. PMID 14018413.
 |
