Chemistry:Lisdexamfetamine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Vyvanse, Tyvense, Elvanse, others |
| Other names | L-Lysine-d-amphetamine; (2S)-2,6-Diamino-N-[(2S)-1-phenylpropan-2-yl]hexanamide N-[(2S)-1-Phenyl-2-propanyl]-L-lysinamide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607047 |
| License data | |
| Pregnancy category |
|
| Dependence liability | Moderate[1][2] |
| Addiction liability | Moderate[1][2] |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 96.4%[6] |
| Protein binding | 20% (as dextroamphetamine)[7] |
| Metabolism | Hydrolysis by enzymes in red blood cells initially, subsequent metabolism follows |
| Metabolites | Dextroamphetamine (and its metabolites) and L-lysine |
| Onset of action | Oral: <2 hours[8][9] |
| Elimination half-life | Lisdexamfetamine: <1 hour[10] Dextroamphetamine: 10–12 h[10][4] |
| Duration of action | 10–12 hours[11][8][9] |
| Excretion | Kidney: ~2% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H25N3O |
| Molar mass | 263.385 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lisdexamfetamine, most commonly sold under the brand name Vyvanse (in the United States and Canada ) and Elvanse (in most European countries) among others, is a stimulant medication that is used to treat attention deficit hyperactivity disorder (ADHD) in children and adults, and for moderate-to-severe binge eating disorder in adults.[12] Lisdexamfetamine is taken by mouth. Its effects generally begin within two hours and last for up to 14 hours.[12] In the United Kingdom, it is usually less preferred to methylphenidate for the treatment of children.[13]
Common side effects of lisdexamfetamine include loss of appetite, anxiety, diarrhea, trouble sleeping, irritability, and nausea.[12] Rare but serious side effects include mania, sudden cardiac death in those with underlying heart problems, and psychosis.[12] It has a high potential for substance abuse per the US Drug Enforcement Administration (DEA).[12] Serotonin syndrome may occur if used with certain other medications.[12] Its use during pregnancy may result in harm to the baby and use during breastfeeding is not recommended by the manufacturer.[14][12][15]
Lisdexamfetamine is an inactive prodrug that works after being converted by the body into dextroamphetamine, a central nervous system (CNS) stimulant.[12][16] Chemically, lisdexamfetamine is composed of the amino acid L-lysine, attached to dextroamphetamine.[17]
Lisdexamfetamine was approved for medical use in the United States in 2007, and in the European Union in 2012.[12][18] In 2021, it was the 69th most commonly prescribed medication in the United States, with more than 9 million prescriptions.[19][20] It is a Class B controlled substance in the United Kingdom and a Schedule II controlled substance in the United States.[14][21]
Uses
Medical
Lisdexamfetamine is used primarily as a treatment for attention deficit hyperactivity disorder (ADHD) and binge eating disorder;[22] it has similar off-label uses as those of other pharmaceutical amphetamines.[11] Individuals over the age of 65 were not commonly tested in clinical trials of lisdexamfetamine for ADHD.[22] According to a 2019 systematic review, lisdexamfetamine was the most effective treatment for adult ADHD.[23] {{#section-h:Amphetamine|Medical}}
Enhancing performance
{{#section-h:Amphetamine|Enhancing performance}}
Available forms
Lisdexamfetamine is available as the dimesylate salt in the form of both oral capsules and chewable tablets.[24] The capsules are available in doses of 10, 20, 30, 40, 50, 60, and 70 mg, while the chewable tablets are available in doses of 10, 20, 30, 40, 50, and 60 mg.[4] These amounts of lisdexamfetamine dimesylate are equivalent to 5.8, 11.6, 17.3, 23.1, 28.9, 34.7, and 40.5 mg lisdexamfetamine free-base, respectively.[24] A dose of 50 mg of lisdexamfetamine dimesylate is approximately equimolar to a 20 mg dose of dextroamphetamine sulfate or to 15 mg dextroamphetamine free-base in terms of the amount of dextroamphetamine contained.[10][25][26] Lisdexamfetamine capsules can be swallowed intact, or they can be opened and mixed into water, yogurt, or applesauce and consumed in that manner.[4][27]
Contraindications
Pharmaceutical lisdexamfetamine is contraindicated in patients with hypersensitivity to amphetamine products or any of the formulation's inactive ingredients.[22] It is also contraindicated in patients who have used a monoamine oxidase inhibitor (MAOI) within the last 14 days.[22][28] Amphetamine products are contraindicated by the United States Food and Drug Administration (USFDA) in people with a history of drug abuse, heart disease, or severe agitation or anxiety, or in those currently experiencing arteriosclerosis, glaucoma, hyperthyroidism, or severe hypertension.[29] However, a European consensus statement on adult ADHD noted that stimulants do not worsen substance misuse in adults with ADHD and comorbid substance use disorder and should not be avoided in these individuals.[30] In any case, the statement noted that immediate-release stimulants should be avoided in those with both ADHD and substance use disorder and that slower-release stimulant formulations like OROS methylphenidate (Concerta) and lisdexamfetamine should be preferred due to their lower misuse potential.[30] Prescribing information approved by the Australian Therapeutic Goods Administration further contraindicates anorexia.[31]
Adverse effects
Products containing lisdexamfetamine have a comparable drug safety profile to those containing amphetamine.[17] The major side effects of lisdexamfetamine in short-term clinical trials (≥5% incidence) have included decreased appetite, insomnia, dry mouth, weight loss, irritability, upper abdominal pain, nausea, vomiting, diarrhea, constipation, increased heart rate, anxiety, dizziness, and feeling jittery.[4][12] Rates of side effects may vary in adults, adolescents, and children.[4] Rare but serious side effects of lisdexamfetamine may include mania, sudden cardiac death in those with underlying heart problems, stimulant psychosis, and serotonin syndrome.[12][4]
Interactions
- Acidifying agents: Drugs that acidify the urine, such as ascorbic acid, increase urinary excretion of dextroamphetamine, thus decreasing the half-life of dextroamphetamine in the body.[22][32]
- Alkalinizing agents: Drugs that alkalinize the urine, such as sodium bicarbonate, decrease urinary excretion of dextroamphetamine, thus increasing the half-life of dextroamphetamine in the body.[22][32]
- CYP2D6 inhibitors: Hydroxylation via the cytochrome P450 enzyme CYP2D6 is the major pathway of metabolism of dextroamphetamine.[33] Potent CYP2D6 inhibitors, such as paroxetine, fluoxetine, bupropion, and duloxetine, among others, may inhibit the metabolism of dextroamphetamine and thereby increase exposure to it.[33][22] Studies characterizing this potential interaction are currently lacking.[33] Concomitant use of lisdexamfetamine with CYP2D6 inhibitors may increase the risk of serotonin syndrome due to greater drug exposure according to the FDA label for lisdexamfetamine.[22]
- Monoamine oxidase inhibitors: Concomitant use of MAOIs and central nervous system stimulants such as lisdexamfetamine can cause a hypertensive crisis.[22]
Pharmacology
Mechanism of action
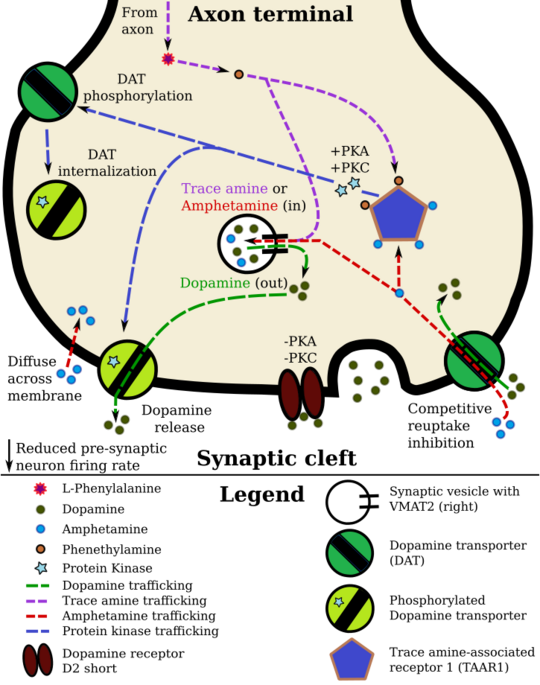
Pharmacodynamics of amphetamine in a dopamine neuron
|
Lisdexamfetamine is an inactive prodrug that is converted in the body to dextroamphetamine, a pharmacologically active compound which is responsible for the drug's activity.[41] After oral ingestion, lisdexamfetamine is broken down by enzymes in red blood cells to form L-lysine, a naturally occurring essential amino acid, and dextroamphetamine.[22] The conversion of lisdexamfetamine to dextroamphetamine is not affected by gastrointestinal pH and is unlikely to be affected by alterations in normal gastrointestinal transit times.[22][42]
The optical isomers of amphetamine, i.e., dextroamphetamine and levoamphetamine, are TAAR1 agonists and vesicular monoamine transporter 2 inhibitors that can enter monoamine neurons;[34][35] this allows them to release monoamine neurotransmitters (dopamine, norepinephrine, and serotonin, among others) from their storage sites in the presynaptic neuron, as well as prevent the reuptake of these neurotransmitters from the synaptic cleft.[34][35]
Lisdexamfetamine was developed with the goal of providing a long duration of effect that is consistent throughout the day, with reduced potential for abuse. The attachment of the amino acid lysine slows down the relative amount of dextroamphetamine available to the blood stream. Because no free dextroamphetamine is present in lisdexamfetamine capsules, dextroamphetamine does not become available through mechanical manipulation, such as crushing or simple extraction. A relatively sophisticated biochemical process is needed to produce dextroamphetamine from lisdexamfetamine.[42] As opposed to Adderall, which contains amphetamine salts in a 3:1 dextro:levo ratio, lisdexamfetamine is a single-enantiomer dextroamphetamine formula.[41][43] Studies conducted show that lisdexamfetamine dimesylate may have less abuse potential than dextroamphetamine and an abuse profile similar to diethylpropion at dosages that are FDA-approved for treatment of ADHD, but still has a high abuse potential when this dosage is exceeded by over 100%.[42]
Pharmacokinetics

{{#section-h:Amphetamine|Pharmacokinetics}}
Chemistry
Lisdexamfetamine is a substituted amphetamine with an amide linkage formed by the condensation of dextroamphetamine with the carboxylate group of the essential amino acid L-lysine.[17] The reaction occurs with retention of stereochemistry, so the product lisdexamfetamine exists as a single stereoisomer. There are many possible names for lisdexamfetamine based on IUPAC nomenclature, but it is usually named as N-[(2S)-1-phenyl-2-propanyl]-L-lysinamide or (2S)-2,6-diamino-N-[(1S)-1-methyl-2-phenylethyl]hexanamide.[45] The condensation reaction occurs with loss of water:
- (S)-PhCH2CH(CH3)NH2 + (S)-HOOCCH(NH2)CH2CH2CH2CH2NH2 → (S,S)-PhCH2CH(CH3)NHC(O)CH(NH2)CH2CH2CH2CH2NH2 + H2O
Amine functional groups are vulnerable to oxidation in air and so pharmaceuticals containing them are usually formulated as salts where this moiety has been protonated. This increases stability, water solubility, and, by converting a molecular compound to an ionic compound, increases the melting point and thereby ensures a solid product.[46] In the case of lisdexamfetamine, this is achieved by reacting with two equivalents of methanesulfonic acid to produce the dimesylate salt, a water-soluble (792 mg mL−1) powder with a white to off-white color.[22]
- PhCH2CH(CH3)NHC(O)CH(NH2)CH2CH2CH2CH2NH2 + 2 CH3SO3H → [PhCH2CH(CH3)NHC(O)CH(NH+3)CH2CH2CH2CH2NH+3][CH3SO−3]2
Comparison to other formulations
Lisdexamfetamine dimesylate is one marketed formulation delivering dextroamphetamine. The following table compares the drug to other amphetamine pharmaceuticals.
| drug | formula | molecular mass [note 1] |
amphetamine base [note 2] |
amphetamine base in equal doses |
doses with equal base content [note 3] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[48][49] | (C9H13N)2•H2SO4 | |||||||||
| amphetamine sulfate[50] | (C9H13N)2•H2SO4 | |||||||||
| Adderall | ||||||||||
| 25% | dextroamphetamine sulfate[48][49] | (C9H13N)2•H2SO4 | ||||||||
| 25% | amphetamine sulfate[50] | (C9H13N)2•H2SO4 | ||||||||
| 25% | dextroamphetamine saccharate[51] | (C9H13N)2•C6H10O8 | ||||||||
| 25% | amphetamine aspartate monohydrate[52] | (C9H13N)•C4H7NO4•H2O | ||||||||
| lisdexamfetamine dimesylate[22] | C15H25N3O•(CH4O3S)2 | |||||||||
| amphetamine base suspension[note 4][53] | C9H13N | |||||||||
History
Lisdexamfetamine was developed by New River Pharmaceuticals, who were bought by Takeda Pharmaceuticals through its acquisition of Shire Pharmaceuticals, shortly before it began being marketed. It was developed with the intention of creating a longer-lasting and less-easily abused version of dextroamphetamine, as the requirement of conversion into dextroamphetamine via enzymes in the red blood cells delays its onset of action, regardless of the route of administration.[54]
In April 2008, the FDA approved lisdexamfetamine for treatment of ADHD in adults.[55] On 4 August 2009, Health Canada approved the marketing of 30 mg and 50 mg capsules of lisdexamfetamine for prescription use.[56]
In January 2015, lisdexamfetamine was approved by the US Food and Drug Administration for treatment of binge eating disorder in adults.[57]
The US Food and Drug Administration gave tentative approval to generic formulations of lisdexamfetamine in 2015.[58] The expiration date for patent protection of lisdexamfetamine in the US was 24 February 2023.[58] The Canadian patent expires 20 years from the filing date of 1 June 2004.[59]
Production quotas for 2016 in the United States were 29,750 kg.[60]
Society and culture
Name
Lisdexamfetamine is the International Nonproprietary Name (INN) and is a contraction of L-lysine-dextroamphetamine.[61]
As of November 2020, lisdexamfetamine is sold under the following brand names: Aduvanz, Elvanse, Juneve, Lisdexamfetamine Dimesylate, Samexid, Tyvense, Venvanse, and Vyvanse.[62]
Research
Depression
Some clinical trials that used lisdexamfetamine as an add-on therapy with a selective serotonin reuptake inhibitor (SSRI) or serotonin-norepinephrine reuptake inhibitor (SNRI) for treatment-resistant depression indicated that this is no more effective than the use of an SSRI or SNRI alone.[63] Other studies indicated that psychostimulants potentiated antidepressants, and were under-prescribed for treatment resistant depression. In those studies, patients showed significant improvement in energy, mood, and psychomotor activity.[64] Clinical guidelines advise caution in the use of stimulants for depression and advise them only as second- or third-line adjunctive agents.[65]
In February 2014, Shire announced that two late-stage clinical trials had found that Vyvanse was not an effective treatment for depression, and development for this indication was discontinued.[66][67] A 2018 meta-analysis of randomized controlled trials of lisdexamfetamine for antidepressant augmentation in people with major depressive disorder—the first to be conducted—found that lisdexamfetamine was not significantly better than placebo in improving Montgomery–Åsberg Depression Rating Scale scores, response rates, or remission rates.[68] However, there was indication of a small effect in improving depressive symptoms that approached trend-level significance.[68] Lisdexamfetamine was well-tolerated in the meta-analysis.[68] The quantity of evidence was limited, with only four trials included.[68] In a subsequent 2022 network meta-analysis, lisdexamfetamine was significantly effective as an antidepressant augmentation for treatment-resistant depression.[65]
Although lisdexamfetamine has shown limited effectiveness in the treatment of depression in clinical trials, a phase II clinical study found that the addition of lisdexamfetamine to an antidepressant improved executive dysfunction in people with mild major depressive disorder but persisting executive dysfunction.[69][70]
While development of lisdexamfetamine for major depressive disorder and bipolar depression was discontinued, the drug remains in phase II clinical trials for treatment of "mood disorders" as of October 2021.[67]
Explanatory notes
- ↑ For uniformity, molecular masses were calculated using the Lenntech Molecular Weight Calculator[47] and were within 0.01g/mol of published pharmaceutical values.
- ↑ Amphetamine base percentage = molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- ↑ dose = (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- ↑ This product (Dyanavel XR) is an oral suspension (i.e., a drug that is suspended in a liquid and taken by mouth) that contains 2.5 mg/mL of amphetamine base.[53] The amphetamine base contains dextro- to levo-amphetamine in a ratio of 3.2:1,[53] which is approximately the ratio in Adderall. The product uses an ion exchange resin to achieve extended release of the amphetamine base.[53]
Reference notes
References
- ↑ 1.0 1.1 "Adderall vs Vyvanse - What's the difference between them?". https://www.drugs.com/medical-answers/adderall-vs-vyvanse-3013810/.
- ↑ 2.0 2.1 "Lisdexamfetamine dimesylate (vyvanse), a prodrug stimulant for attention-deficit/hyperactivity disorder". P & T 35 (5): 273–287. May 2010. PMID 20514273.
- ↑ "Australian Product Information Vyanse® (Lisdexamfetamine dimesilate)". https://www.tga.gov.au/sites/default/files/auspar-lisdexamfetamine-dimesilate-180515-pi.pdf.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "Vyvanse- lisdexamfetamine dimesylate capsule VYVANSE- lisdexamfetamine dimesylate tablet, chewable". 10 March 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=704e4378-ca83-445c-8b45-3cfa51c1ecad.
- ↑ "List of nationally authorised medicinal products : Active substance(s): lisdexamfetamine : Procedure No. PSUSA/00010289/202002". https://www.ema.europa.eu/documents/psusa/lisdexamfetamine-list-nationally-authorised-medicinal-products-psusa/00010289/202002_en.pdf.
- ↑ "Public Assessment Report Decentralised Procedure". MHRA. p. 14. http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con261790.pdf.
- ↑ Template:Cite DrugBank
- ↑ 8.0 8.1 Millichap JG, ed (2010). "Chapter 9: Medications for ADHD". Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. pp. 112. ISBN 9781441913968. "
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h]" - ↑ 9.0 9.1 "Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder". Postgraduate Medicine 120 (3): 69–88. September 2008. doi:10.3810/pgm.2008.09.1909. PMID 18824827. "Onset of efficacy was earliest for d-MPH-ER at 0.5 hours, followed by d, l-MPH-LA at 1 to 2 hours, MCD at 1.5 hours, d, l-MPH-OR at 1 to 2 hours, MAS-XR at 1.5 to 2 hours, MTS at 2 hours, and LDX at approximately 2 hours. ... MAS-XR, and LDX have a long duration of action at 12 hours postdose".
- ↑ 10.0 10.1 10.2 "Lisdexamfetamine Dimesylate: Prodrug Delivery, Amphetamine Exposure and Duration of Efficacy". Clinical Drug Investigation 36 (5): 341–356. May 2016. doi:10.1007/s40261-015-0354-y. PMID 27021968.
- ↑ 11.0 11.1 "Lisdexamfetamine". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. March 2017. pp. 379–384. ISBN 9781108228749.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 "Lisdexamfetamine Dimesylate Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/lisdexamfetamine-dimesylate.html.
- ↑ "Attention deficit hyperactivity disorder: diagnosis and management". 14 March 2018. https://www.nice.org.uk/guidance/ng87/chapter/recommendations#medication.
- ↑ 14.0 14.1 British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 348–349. ISBN 9780857113382.
- ↑ "Lisdexamfetamine (Vyvanse) Use During Pregnancy". https://www.drugs.com/pregnancy/lisdexamfetamine.html.
- ↑ "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology 27 (6): 479–496. June 2013. doi:10.1177/0269881113482532. PMID 23539642.
- ↑ 17.0 17.1 17.2 "Lisdexamfetamine". Paediatric Drugs 9 (2): 129–135; discussion 136–138. 2007. doi:10.2165/00148581-200709020-00007. PMID 17407369.
- ↑ "Shire's ADHD amphetamine wins British backing". Reuters. 12 December 2012. https://www.reuters.com/article/us-shire-adhd-idUKBRE8BH0X820121218/.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Lisdexamfetamine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Lisdexamfetamine.
- ↑ Drugs of Abuse. Drug Enforcement Administration • U.S. Department of Justice. 2017. p. 22. https://www.dea.gov/sites/default/files/2018-06/drug_of_abuse.pdf. Retrieved 16 April 2019.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 22.12 Cite error: Invalid
<ref>tag; no text was provided for refs namedUSVyvanselabel - ↑ "Efficacy, Acceptability, and Tolerability of Lisdexamfetamine, Mixed Amphetamine Salts, Methylphenidate, and Modafinil in the Treatment of Attention-Deficit Hyperactivity Disorder in Adults: A Systematic Review and Meta-analysis". The Annals of Pharmacotherapy 53 (2): 121–133. February 2019. doi:10.1177/1060028018795703. PMID 30117329.
- ↑ 24.0 24.1 "Drugs@FDA: FDA-Approved Drugs". https://www.accessdata.fda.gov/scripts/cder/daf/.
- ↑ 25.0 25.1 25.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid28936175 - ↑ "Elvanse Adult 30mg Hard Capsules". https://www.medicines.org.uk/emc/product/6828/smpc. "2. Qualitative and quantitative composition. 30 mg Capsules: Each capsule contains 30 mg lisdexamfetamine dimesylate, equivalent to 8.9 mg of dexamfetamine. 50 mg Capsules: Each capsule contains 50 mg lisdexamfetamine dimesylate, equivalent to 14.8 mg of dexamfetamine. 70 mg Capsules: Each capsule contains 70 mg lisdexamfetamine dimesylate, equivalent to 20.8 mg of dexamfetamine."
- ↑ "Relative Bioavailabilities of Lisdexamfetamine Dimesylate and D-Amphetamine in Healthy Adults in an Open-Label, Randomized, Crossover Study After Mixing Lisdexamfetamine Dimesylate With Food or Drink". Ther Drug Monit 38 (6): 769–776. December 2016. doi:10.1097/FTD.0000000000000343. PMID 27661399.
- ↑ "Amphetamine (PIM 934)". International Programme on Chemical Safety. http://www.inchem.org/documents/pims/pharm/pim934.htm.
- ↑ "Adderall XR Prescribing Information". Shire US Inc.. December 2013. pp. 4–6. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf.
- ↑ 30.0 30.1 "Updated European Consensus Statement on diagnosis and treatment of adult ADHD". European Psychiatry 56: 14–34. February 2019. doi:10.1016/j.eurpsy.2018.11.001. PMID 30453134.
- ↑ "Dexamphetamine tablets". https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2009-PI-00828-3.
- ↑ 32.0 32.1 "Adderall XR Prescribing Information". Shire US Inc.. December 2013. pp. 8–10. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf.
- ↑ 33.0 33.1 33.2 "Clinically Significant Drug-Drug Interactions with Agents for Attention-Deficit/Hyperactivity Disorder". CNS Drugs 33 (12): 1201–1222. December 2019. doi:10.1007/s40263-019-00683-7. PMID 31776871.
- ↑ 34.0 34.1 34.2 34.3 34.4 34.5 34.6 "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry 116 (2): 164–176. January 2011. doi:10.1111/j.1471-4159.2010.07109.x. PMID 21073468.
- ↑ 35.0 35.1 35.2 35.3 "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Annals of the New York Academy of Sciences 1216 (1): 86–98. January 2011. doi:10.1111/j.1749-6632.2010.05906.x. PMID 21272013. Bibcode: 2011NYASA1216...86E. "VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).".
- ↑ "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia 6 (3): 123–148. August 2016. doi:10.1016/j.baga.2016.02.001. PMID 27141430. "Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.".
- ↑ "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. July 2011. doi:10.3389/fnsys.2011.00056. PMID 21772817. "Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.".
- ↑ "TAAR1". GenAtlas. University of Paris. 28 January 2012. http://genatlas.medecine.univ-paris5.fr/fiche.php?symbol=TAAR1. Retrieved 29 May 2014. " • tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)"
- ↑ "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron 83 (2): 404–416. July 2014. doi:10.1016/j.neuron.2014.05.043. PMID 25033183. "AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH".
- ↑ "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. September 2013. doi:10.1016/j.tips.2013.07.005. PMID 23968642. "AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].".
- ↑ 41.0 41.1 Template:Cite DrugBank
- ↑ 42.0 42.1 42.2 "Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse". Journal of Psychopharmacology 23 (4): 419–427. June 2009. doi:10.1177/0269881109103113. PMID 19329547.
- ↑ "Adderall XR Prescribing Information". pp. 1–18. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s024lbl.pdf.
- ↑ "Effects of lisdexamfetamine on plasma steroid concentrations compared with d-amphetamine in healthy subjects: A randomized, double-blind, placebo-controlled study". The Journal of Steroid Biochemistry and Molecular Biology 186: 212–225. February 2019. doi:10.1016/j.jsbmb.2018.10.016. PMID 30381248. https://edoc.unibas.ch/69588/1/20190220112204_5c6d2a4cdf9fd.pdf.
- ↑ "Lisdexamfetamine". Royal Society of Chemistry. 2015. http://www.chemspider.com/Chemical-Structure.9772458.html.
- ↑ Pharmaceutical Salts: Properties, Selection, and Use (2nd ed.). John Wiley & Sons. 2011. ISBN 978-3-90639-051-2.
- ↑ "Molecular Weight Calculator". Lenntech. http://www.lenntech.com/calculators/molecular/molecular-weight-calculator.htm. Retrieved 19 August 2015.
- ↑ 48.0 48.1 "Dextroamphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491568. Retrieved 19 August 2015.
- ↑ 49.0 49.1 "D-amphetamine sulfate". Tocris. 2015. http://www.tocris.com/dispprod.php?ItemId=5305#.VXpspvlViko. Retrieved 19 August 2015.
- ↑ 50.0 50.1 "Amphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491599. Retrieved 19 August 2015.
- ↑ "Dextroamphetamine Saccharate". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491576. Retrieved 19 August 2015.
- ↑ "Amphetamine Aspartate". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491591. Retrieved 19 August 2015.
- ↑ "Lisdexamfetamine dimesylate: a prodrug stimulant for the treatment of ADHD in children and adults". CNS Spectrums 15 (5): 315–325. May 2010. doi:10.1017/S1092852900027541. PMID 20448522. https://digitalcommons.wustl.edu/open_access_pubs/3506.
- ↑ "FDA Adult Approval of Vyvanse – FDA Label and Approval History". http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/021977s001ltr.pdf.
- ↑ "Drug Product Database". Health Canada. 25 April 2012. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=80843.
- ↑ "FDA expands uses of Vyvanse to treat binge-eating disorder". U.S. Food and Drug Administration (FDA) (Press release). 30 January 2015. Archived from the original on 26 January 2018. Retrieved 19 March 2023.
- ↑ 58.0 58.1 "Tentative approval". 3 March 2023. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/202802Orig1s000TAltr.pdf.
- ↑ Government of Canada, Innovation. "Canadian Patent Database / Base de données sur les brevets canadiens" (in en). https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2527646/summary.html.
- ↑ "DEA Office of Diversion Control". DEA. http://www.deadiversion.usdoj.gov/quotas/quota_history.pdf.
- ↑ "Alternative pharmacological strategies for adult ADHD treatment: a systematic review". Expert Review of Neurotherapeutics 16 (2): 131–144. 2016. doi:10.1586/14737175.2016.1135735. PMID 26693882.
- ↑ "Lisdexamfetamine international brands". Drugs.com. https://www.drugs.com/international/lisdexamfetamine.html.
- ↑ "Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs". Biochemical Pharmacology 95 (2): 81–97. May 2015. doi:10.1016/j.bcp.2015.03.011. PMID 25813654.
- ↑ "Psychostimulants in the therapy of treatment-resistant depression Review of the literature and findings from a retrospective study in 65 depressed patients". Dialogues in Clinical Neuroscience 1 (3): 165–174. December 1999. doi:10.31887/DCNS.1999.1.3/gstotz. PMID 22034135.
- ↑ 65.0 65.1 "Augmentation strategies for treatment resistant major depression: A systematic review and network meta-analysis". Journal of Affective Disorders 302: 385–400. April 2022. doi:10.1016/j.jad.2021.12.134. PMID 34986373.
- ↑ "UPDATE 2-Shire scraps Vyvanse for depression after failed trials". Reuters. 7 February 2014. http://uk.reuters.com/article/shire-vyvanse-idUKL2N0LB28F20140207.
- ↑ 67.0 67.1 "Lisdexamfetamine - Shionogi/Takeda". https://adisinsight.springer.com/drugs/800020876. "Clinical development is underway in the US, for mood disorders in children and adolescents for binge eating disorder and ADHD."
- ↑ 68.0 68.1 68.2 68.3 "Efficacy and tolerability of lisdexamfetamine as an antidepressant augmentation strategy: A meta-analysis of randomized controlled trials". Journal of Affective Disorders 226: 294–300. January 2018. doi:10.1016/j.jad.2017.09.041. PMID 29028590.
- ↑ "Translational Medicine Strategies in Drug Development for Mood Disorders". Translational Medicine in CNS Drug Development. Handbook of Behavioral Neuroscience. 29. Elsevier. 2019. pp. 333–347. doi:10.1016/B978-0-12-803161-2.00023-0. ISBN 9780128031612.
- ↑ "Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder". Neuropsychopharmacology 39 (6): 1388–1398. May 2014. doi:10.1038/npp.2013.334. PMID 24309905.
 |