Chemistry:Thozalinone
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Tozalinone, Thozalinon |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
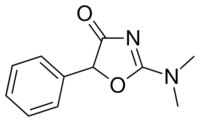
| Formula | C11H12N2O2 |
| Molar mass | 204.229 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Thozalinone (USAN) (brand name Stimsen; former developmental code name CL-39808) is a psychostimulant that has been used as an antidepressant in Europe.[1][2][3][4][5] It has also been trialed as an anorectic.[6] Thozalinone is described as a "dopaminergic stimulant",[7] and likely acts via inducing the release of dopamine and to a minimal extent norepinephrine; similar to analogue pemoline, it is reportedly devoid of abuse potential unlike other dopaminergic psychostimulants.[2][7][8]
Synthesis
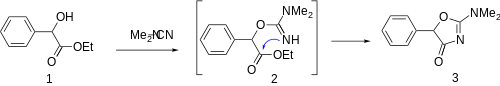
Sodium hydride is used as a strong base to abstract the alcohol proton in ethyl mandelate [774-40-3] (1); addition of the oxyanion to dimethylcyanamide [1467-79-4] gives the intermediate (2). Intramolecular cyclization then occurs giving Thozalinone (3).
Notes
- In treatment of Parkinsonism: W. D. Gray, C. E. Edward, U.S. Patent 3,665,075 (1972 to Am. Cyanamid).
- Pharmacological studies:[11]
See also
References
- ↑ The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 435–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA435.
- ↑ 2.0 2.1 "Some pharmacologic properties of thozalinone, a new excitant". Toxicology and Applied Pharmacology 7 (4): 566–78. July 1965. doi:10.1016/0041-008X(65)90042-6. PMID 4378772.
- ↑ Dictionary of organic compounds. London: Chapman & Hall. 1996. ISBN 0-412-54090-8. https://books.google.com/books?id=C3Uo1co4Wv0C&q=thozalinone&pg=PA2539. Retrieved 2021-05-31.
- ↑ Merck index on CD-ROM: Windows. London: Chapman & Hall EPD. 1998. ISBN 0-412-82910-X.
- ↑ "A double-blind study of thozalinone (C1 39,808) in depressed outpatients". Current Therapeutic Research, Clinical and Experimental 8 (12): 621–2. December 1966. PMID 4962734.
- ↑ "[Clinical trial of Stimsem Thozalinone in the treatment of obese patients]" (in pt). Revista Brasileira de Medicina 28 (9): 475–8. September 1971. PMID 5139648.
- ↑ 7.0 7.1 "Detection of dopaminergic supersensitivity induced by neuroleptic drugs in mice". Drug and Chemical Toxicology 3 (2): 237–47. 1980. doi:10.3109/01480548009108286. PMID 6112126.
- ↑ "Inhibition of dopaminergic agonist-induced gnawing behavior by neuroleptic drugs in mice". Drug and Chemical Toxicology 8 (6): 495–502. 1985. doi:10.3109/01480548509041072. PMID 2868876.
- ↑ "2-Amino-2-oxazolin-4-ones. I. Synthesis.". The Journal of Organic Chemistry 27 (5): 1679–1685. May 1962. doi:10.1021/jo01052a047.
- ↑ "Compounds related to pemoline. 2-amino-5-aryl-4-oxo-2-oxazolines". Acta Pharmaceutica Suecica 5 (1): 15–22. February 1968. PMID 4386169.
- ↑ "Behavioral facilitation: The interaction of imipramine and desipramine with amphetamine, alpha-pipradrol, methylphenidate, and thozalinone". Psychopharmacologia 12 (4): 338–345. 1968. doi:10.1007/BF00401412. PMID 4385109.
 |
