Chemistry:Osmium octafluoride
From HandWiki
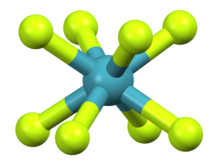 Approximate geometry predicted computationally
| |
| Names | |
|---|---|
| IUPAC name
Octafluoroosmium[1]
| |
| Other names
Osmium(VIII) fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| OsF 8 | |
| Molar mass | 342.22 g·mol−1 |
| Structure | |
| C2/c (4 GPa) R3 (240 GPa)[2] | |
| Related compounds | |
Related compounds
|
Xenon octafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Osmium octafluoride is an inorganic chemical compound of osmium metal and fluorine with the chemical formula OsF
8.[3][4] Some sources consider it to be a still hypothetical compound.[5] An early report of the synthesis of OsF
8 was much later shown to be a mistaken identification of OsF
6.[6] Theoretical analysis indicates OsF
8 would have an approximately square antiprismatic molecular geometry.[7]
Potential synthesis
Rapid cooling of fluorine and osmium reaction products:[8]
- Os + 4 F
2 → OsF
8
References
- ↑ "Octafluoroosmium". https://pubchem.ncbi.nlm.nih.gov/compound/57496620.
- ↑ Lin, Jianyan; Du, Xin; Rahm, Martin; Yu, Hong; Xu, Haiyang; Yang, Guochun (25 March 2020). "Exploring the Limits of Transition-Metal Fluorination at High Pressures". Angewandte Chemie International Edition 59 (23): 9155–9162. doi:10.1002/anie.202002339. ISSN 1433-7851. PMID 32150319.
- ↑ (in en) Routledge German Dictionary of Chemistry and Chemical Technology Worterbuch Chemie und Chemische Technik: Vol 1: German-English. Routledge. 17 June 2014. p. 481. ISBN 978-1-136-76231-4. https://books.google.com/books?id=1BnYAwAAQBAJ&dq=Osmium+octafluoride&pg=PA481. Retrieved 30 March 2023.
- ↑ Macintyre, Jane E. (23 July 1992) (in en). Dictionary of Inorganic Compounds. CRC Press. p. 3247. ISBN 978-0-412-30120-9. https://books.google.com/books?id=9eJvoNCSCRMC&dq=Osmium+octafluoride&pg=PA3247. Retrieved 30 March 2023.
- ↑ Haupt, Axel (22 March 2021) (in en). Organic and Inorganic Fluorine Chemistry: Methods and Applications. Walter de Gruyter GmbH & Co KG. p. 258. ISBN 978-3-11-065933-7. https://books.google.com/books?id=5fwkEAAAQBAJ&dq=Osmium+octafluoride&pg=PA258. Retrieved 30 March 2023.
- ↑ Riedel, S.; Kaupp, M. (30 Jul 2009). "The highest oxidation states of the transition metal elements". Coordination Chemistry Reviews 253 (5–6): 606–624. doi:10.1016/j.ccr.2008.07.014.
- ↑ Riedel, Sebastian; Kaupp, Martin (2006). "Where Is the Limit of Highly Fluorinated High-Oxidation-State Osmium Species?". Inorg. Chem. 45 (26): 10497–10502. doi:10.1021/ic061054y. PMID 17173405.
- ↑ Satya, Prakash (2013) (in en). Advanced Chemistry of Rare Elements. S. Chand Publishing. p. 612. ISBN 978-81-219-4254-6. https://books.google.com/books?id=WB_4DwAAQBAJ&dq=Osmium+octafluoride+osf8&pg=PA612. Retrieved 30 March 2023.
 |

