Chemistry:PSB-10
 | |
| Clinical data | |
|---|---|
| Other names | PSB-10 |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| Chemical and physical data | |
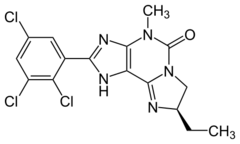
| Formula | C16H14Cl3N5O |
| Molar mass | 398.67 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
PSB-10 is a drug which acts as a selective antagonist[1] for the adenosine A3 receptor (ki value at human A3 receptor is 0.44 nM), with high selectivity over the other three adenosine receptor subtypes (ki values at human A1, A2A and A2B receptors are 4.1, 3.3 and 30 μM). Further pharmacological experiments in a [35S]GTPγS binding assay using hA3-CHO-cells indicated that PSB-10 acts as an inverse agonist (IC50 = 4 nM). It has been shown to produce antiinflammatory effects in animal studies.[2] Simple xanthine derivatives such as caffeine and DPCPX have generally low affinity for the A3 subtype and must be extended by expanding the ring system and adding an aromatic group to give high A3 affinity and selectivity.[3] The affinity towards adenosine A3 subtype was measured against the radioligand PSB-11.[4]
References
- ↑ "2-Phenylimidazo[2,1-ipurin-5-ones: structure-activity relationships and characterization of potent and selective inverse agonists at Human A3 adenosine receptors"]. Bioorg Med Chem 11 (3): 347–56. 2003. doi:10.1016/S0968-0896(02)00456-X. PMID 12517430.
- ↑ "Adenosine receptor subtype-selective antagonists in inflammation and hyperalgesia". Naunyn-Schmiedeberg's Archives of Pharmacology 377 (1): 65–76. March 2008. doi:10.1007/s00210-007-0252-9. PMID 18188542.
- ↑ Müller CE (2003). "Medicinal chemistry of adenosine A3 receptor ligands". Current Topics in Medicinal Chemistry 3 (4): 445–62. doi:10.2174/1568026033392174. PMID 12570761. http://www.bentham-direct.org/pages/content.php?CTMC/2003/00000003/00000004/0008R.SGM. Retrieved April 28, 2020.
- ↑ "[(3)H]8-Ethyl-4-methyl-2-phenyl-(8R)-4,5,7,8-tetrahydro-1H-imidazo[2,1-i]-purin-5-one ([(3)H]PSB-11), a novel high-affinity antagonist radioligand for human A(3) adenosine receptors.". Bioorg Med Chem Lett 12 (3): 501–3. 2002. doi:10.1016/S0960-894X(01)00785-5. PMID 11814828.
 |
