Chemistry:ZM-241,385
From HandWiki
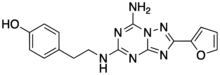
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-(2-{[7-Amino-2-(furan-2-yl)[1,2,4]triazolo[1,5-a][1,3,5]triazin-5-yl]amino}ethyl)phenol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H15N7O2 | |
| Molar mass | 337.343 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
ZM-241,385 is a high affinity antagonist ligand selective for the adenosine A2A receptor.[1]
In animal models, ZM-241,385 has been shown to protect against beta amyloid neurotoxicity and therefore may be useful as a treatment for Alzheimer's disease.[2] ZM-241,385 has also been shown to enhance L-DOPA derived dopamine release and therefore may be useful in the treatment of Parkinson's disease.[3]
References
- ↑ "125I-4-(2-(7-amino-2-(2-furyl)(1,2,4)triazolo(2,3-a)(1,3,5) triazin-5-yl-amino)ethyl)phenol, a high affinity antagonist radioligand selective for the A2a adenosine receptor". Molecular Pharmacology 48 (6): 970–4. December 1995. PMID 8848012. PMC 3479638. http://molpharm.aspetjournals.org/cgi/content/abstract/48/6/970.
- ↑ "Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity". British Journal of Pharmacology 138 (7): 1207–9. April 2003. doi:10.1038/sj.bjp.0705185. PMID 12711619.
- ↑ "Striatal adenosine A(2A) receptor blockade increases extracellular dopamine release following l-DOPA administration in intact and dopamine-denervated rats". Neuropharmacology 47 (3): 414–26. September 2004. doi:10.1016/j.neuropharm.2004.04.018. PMID 15275831.
External links
ZM+241385 at the US National Library of Medicine Medical Subject Headings (MeSH)
 |

