Chemistry:KN-62
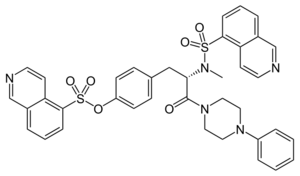
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-[(2S)-2-(N-Methylisoquinoline-5-sulfonamido)-3-oxo-3-(4-phenylpiperazin-1-yl)propyl]phenyl isoquinoline-5-sulfonate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | C063302 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C38H35N5O6S2 | |
| Molar mass | 721.84 g/mol |
| Boiling point | 964.7±75.0 °C at 760 mmHg |
| Hazards | |
| Flash point | 537.3±37.1 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
KN-62 is a derivative of isoquinolinesulfonamide, it is a selective, specific and cell permeable inhibitor of Ca2+/calmodulin-dependent kinase type II (CaMK II) with IC50 of 900nM,[1] charactered by hydrophobicity. KN-62 also potently inhibits the purinergic receptor P2X7.[2]
Inhibitory mechanism on CaMK II
KN-62 blocks the combination of CaM and CaMK II by binding directly to the calmodulin binding site of the enzyme, disenables CaMK II's autophosphorylation, consequently leading inactivation. Kinetic analysis exhibits that this inhibitory effect of KN-62 is competitive with respect to calmodulin.[3] Since KN-62 binds to the calmodulin binding site of CaMK II, KN-62 doesn't inhibit activity of autophosphorylated CaMK II.
Besides, KN-62 also acts as a potent non-competitive antagonist at the purinergic receptor P2RX7 with IC50 of 15nM.
References
- ↑ "avtivity in vitro and in vivo of KN-62". selleck chemicals. http://www.selleckchem.com/products/kn-62.html.
- ↑ Humphreys, BD; Virginio, C; Surprenant, A; Rice, J; Dubyak, GR (July 1998). "Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues.". Molecular Pharmacology 54 (1): 22–32. doi:10.1124/mol.54.1.22. PMID 9658186.
- ↑ "KN-62, a specific Ca++/calmodulin-dependent protein kinase inhibitor, reversibly depresses the rate of beating of cultured fetal mouse cardiac myocytes.". J Pharmacol Exp Ther 270 (3): 1319–24. Sep 1994. PMID 7932185.
 |
