Chemistry:SCH-442,416
From HandWiki
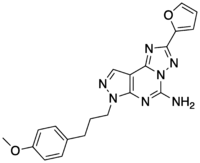
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-(Furan-2-yl)-7-[3-(4-methoxyphenyl)propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine | |
| Other names
SCH-442,416
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H19N7O2 | |
| Molar mass | 389.410 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
SCH-442,416 is a highly selective adenosine A2a subtype receptor antagonist. It is widely used in its 11C radiolabelled form to map the distribution of A2a receptors in the brain, where they are mainly found in the striatum, nucleus accumbens, and olfactory tubercle.[1] Given its distribution in the brain, A2a receptors have been investigated for the treatment of various neurological disorders, and SCH-442,416 has shown promise in treatment of depression,[2] Parkinson's disease,[3] and catalepsy.[4]
References
- ↑ "In vivo imaging of adenosine A2A receptors in rat and primate brain using [11C]SCH442416". European Journal of Nuclear Medicine and Molecular Imaging 32 (4): 405–13. April 2005. doi:10.1007/s00259-004-1688-5. PMID 15549298.
- ↑ "Adenosine A2A receptors and depression". Neurology 61 (11 Suppl 6): S82-7. December 2003. doi:10.1212/01.wnl.0000095220.87550.f6. PMID 14663017.
- ↑ "Synergistic effects of adenosine A2A antagonist and L-DOPA on rotational behaviors in 6-hydroxydopamine-induced hemi-Parkinsonian mouse model". Journal of Pharmacological Sciences 103 (3): 329–32. March 2007. doi:10.1254/jphs.scz070058. PMID 17341841.
- ↑ "Brain adenosine A2A receptor occupancy by a novel A1/A2A receptor antagonist, ASP5854, in rhesus monkeys: relationship to anticataleptic effect". Journal of Nuclear Medicine 49 (7): 1183–8. July 2008. doi:10.2967/jnumed.108.051474. PMID 18552135.
External links
 |

