Chemistry:Promethium(III) chloride
 Glowing powder mixture of promethium(III) chloride and zinc sulfide
| |
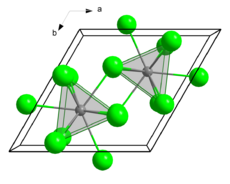 Crystal structure
| |
| Names | |
|---|---|
| Other names
Promethium chloride; Promethium trichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Cl3Pm | |
| Molar mass | 251 g·mol−1 |
| Density | 4.19 g/cm3 (calc., XRD)[1] |
| Melting point | 655 °C (1,211 °F; 928 K)[2] |
| Structure | |
| Trigonal, hP8 | |
| P63/m, No. 176[1] | |
| Related compounds | |
Other anions
|
Promethium(III) oxide |
Other cations
|
Neodymium(III) chloride, Samarium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Promethium(III) chloride is a chemical compound of promethium and chlorine with the formula PmCl3. It is an ionic, water soluble, crystalline salt that glows in the dark with a pale blue or green light due to promethium's intense radioactivity.
Preparation
Promethium(III) chloride is obtained from promethium(III) oxide by heating it in a stream of dry HCl at 580 °C.[3]
Properties
Promethium(III) chloride is a purple solid with a melting point of 655 °C.[4] It crystallizes in the hexagonal crystal system (NdCl3 type) with the lattice parameters a = 739 pm and c = 421 pm with two formula units per unit cell and thus a calculated density of 4.19 g·cm−3.[5][6] When PmCl3 is heated in the presence of H2O, the pale pink colored promethium(III) oxychloride (PmOCl) is obtained.[5][7]
Applications
Promethium(III) chloride (with 147Pm) has been used to generate long-lasting glow in signal lights and buttons. This application relied on the unstable nature of promethium, which emitted beta radiation (electrons) with a half-life of several years. The electrons were absorbed by a phosphor, generating visible glow.[8] Unlike many other radioactive nuclides, promethium-147 does not emit alpha particles that would degrade the phosphor.[9]
References
- ↑ 1.0 1.1 Weigel, F.; Scherer, V. (1967). "Die Chemie des Promethiums". Radiochimica Acta 7. doi:10.1524/ract.1967.7.1.40.
- ↑ Haynes, William M., ed (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.84. ISBN 1439855110.
- ↑ Gmelins Handbuch der anorganischen Chemie, System No. 39, p. 61–62.
- ↑ Wiberg, Egon; Wiberg, Nils (2007). Holleman, Arnold F.. ed. Lehrbuch der anorganischen Chemie (102., stark umgearbeitete und verbesserte Auflage ed.). Berlin New York: Walter de Gruyter. ISBN 978-3-11-017770-1.
- ↑ 5.0 5.1 Weigel: Die Chemie des Promethiums, p. 588–589.
- ↑ Gmelins Handbuch der anorganischen Chemie, System No. 39, p. 181.
- ↑ Gmelins Handbuch der anorganischen Chemie, System No. 39, p. 31.
- ↑ Haynes, William M., ed (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.28. ISBN 1439855110.
- ↑ Lavrukhina, Avgusta Konstantinovna; Pozdnyakov, Aleksandr Aleksandrovich (1966) (in Russian). Аналитическая химия технеция, прометия, астатина и франция [Analytical Chemistry of Technetium, Promethium, Astatine, and Francium]. Nauka. p. 118.
 |

