Chemistry:Samarium(III) chloride

| |
| |
| Names | |
|---|---|
| IUPAC name
samarium(III) chloride
| |
| Other names
samarium trichloride
trichlorosamarium | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| SmCl3 | |
| Molar mass | 256.76 g/mol (anhydrous) 364.80 g/mol (hexahydrate) |
| Appearance | pale yellow solid (anhydrous)
cream-coloured solid (hexahydrate) |
| Density | 4.46 g/cm3 (anhydrous)
2.383 g/cm3 (hexahydrate) |
| Melting point | 682 °C (1,260 °F; 955 K) |
| Boiling point | decomposes |
| 92.4 g/100 mL (10 °C) | |
| Structure | |
| hexagonal, hP8 | |
| P63/m, No. 176 | |
| Tricapped trigonal prismatic (nine-coordinate) | |
| Hazards | |
| Main hazards | Irritant |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362 | |
| Related compounds | |
Other anions
|
Samarium(III) fluoride Samarium(III) bromide Samarium(III) oxide |
Other cations
|
Samarium(II) chloride Promethium(III) chloride Europium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Samarium(III) chloride, also known as samarium trichloride, is an inorganic compound of samarium and chloride. It is a pale yellow salt that rapidly absorbs water to form a hexahydrate, SmCl3.6H2O.[1] The compound has few practical applications but is used in laboratories for research on new compounds of samarium.
Structure
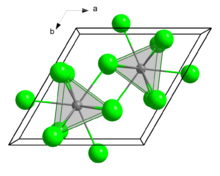
Like several related chlorides of the lanthanides and actinides, SmCl3 crystallises in the UCl3 motif. The Sm3+ centres are nine-coordinate, occupying trigonal prismatic sites with additional chloride ligands occupying the three square faces.
Preparation and reactions
SmCl3 is prepared by the "ammonium chloride" route, which involves the initial synthesis of (NH4)2[SmCl5]. This material can be prepared from the common starting materials at reaction temperatures of 230 °C from samarium oxide:[2]
- 10 NH4Cl + Sm2O3 → 2 (NH4)2[SmCl5] + 6 NH3 + 3 H2O
The pentachloride is then heated to 350-400 °C resulting in evolution of ammonium chloride and leaving a residue of the anhydrous trichloride:
- (NH4)2[SmCl5] → 2 NH4Cl + SmCl3
It can also be prepared from samarium metal and hydrochloric acid.[3][4]
- 2 Sm + 6 HCl → 2 SmCl3 + 3 H2
Aqueous solutions of samarium(III) chloride can be prepared by dissolving metallic samarium or samarium carbonate in hydrochloric acid.
Samarium(III) chloride is a moderately strong Lewis acid, which ranks as "hard" according to the HSAB concept. Aqueous solutions of samarium chloride can be used to prepare samarium trifluoride:
- SmCl3 + 3 KF → SmF3 + 3 KCl
Uses
Samarium(III) chloride is used for the preparation of samarium metal, which has a variety of uses, notably in magnets. Anhydrous SmCl3 is mixed with sodium chloride or calcium chloride to give a low melting point eutectic mixture. Electrolysis of this molten salt solution gives the free metal.[5]
In laboratory
Samarium(III) chloride can also be used as a starting point for the preparation of other samarium salts. The anhydrous chloride is used to prepare organometallic compounds of samarium, such as bis(pentamethylcyclopentadienyl)alkylsamarium(III) complexes.[6]
References
- ↑ F. T. Edelmann, P. Poremba (1997). W. A. Herrmann. ed. Synthetic Methods of Organometallic and Inorganic Chemistry. 6. Stuttgart: Georg Thieme Verlag.
- ↑ Meyer, G. (1989). "The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides—The Example of Ycl 3". The Ammonium Chloride Route to Anhydrous Rare Earth Chlorides-The Example of YCl3. Inorganic Syntheses. 25. pp. 146–150. doi:10.1002/9780470132562.ch35. ISBN 978-0-470-13256-2.
- ↑ L. F. Druding, J. D. Corbett (1961). "Lower Oxidation States of the Lanthanides. Neodymium(II) Chloride and Iodide". J. Am. Chem. Soc. 83 (11): 2462–2467. doi:10.1021/ja01472a010.
- ↑ J. D. Corbett (1973). "Reduced Halides of the Rare Earth Elements". Rev. Chim. Minérale 10: 239.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. ISBN 978-0-08-022057-4. https://books.google.com/books?id=OezvAAAAMAAJ&q=0-08-022057-6&dq=0-08-022057-6&source=bl&ots=m4tIRxdwSk&sig=XQTTjw5EN9n5z62JB3d0vaUEn0Y&hl=en&sa=X&ei=UoAWUN7-EM6ziQfyxIDoCQ&ved=0CD8Q6AEwBA.
- ↑ G. A. Molander, E. D. Dowdy (1999). Shu Kobayashi. ed. Lanthanides: Chemistry and Use in Organic Synthesis. Berlin: Springer-Verlag. pp. 119–154. ISBN 3-540-64526-8. https://archive.org/details/springer_10.1007-3-540-69801-9.
 |

