Chemistry:Thulium(III) chloride
| |
| Names | |
|---|---|
| IUPAC name
Thulium(III) chloride
| |
| Other names
Thulium chloride, thulium trichloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| TmCl3 | |
| Molar mass | 275.292 g/mol |
| Appearance | yellow crystals |
| Density | 3.98 g/cm3 |
| Melting point | 824 °C (1,515 °F; 1,097 K) |
| Boiling point | 1,490 °C (2,710 °F; 1,760 K) |
| heptahydrate: very soluble | |
| Solubility | heptahydrate: very soluble in ethanol[1] |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| 6[2] | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-966.6 kJ/mol[3] |
| Hazards | |
| Main hazards | Irritant |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Other anions
|
Thulium(III) oxide |
Other cations
|
Erbium(III) chloride Ytterbium(III) chloride Thulium(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
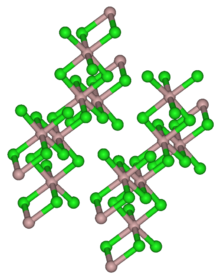
Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral thulium ions.[5] It has been used as a starting material for some exotic nanostructures prepared for NIR photocatalysis.[6][7]
Preparation
Thulium(III) chloride can be obtained by reacting thulium(III) oxide or thulium(III) carbonate and ammonium chloride:[8]
- Tm
2O
3 + 6 NH
4Cl → 2 TmCl
3 + 6 NH
3 + 2 H
2O
The hexahydrate of thulium(III) chloride can be obtained by adding thulium(III) oxide to concentrated hydrochloric acid.[1][8]
- 2 Tm + 6 HCl → 2 TmCl
3 + 3 H
2
Thulium(III) chloride can also be obtained by directly reacting thulium and chlorine:[9]
- 2 Tm + 3 Cl
2 → 2 TmCl
3
Properties
Thulium(III) chloride is a light yellow powder. Its hexahydrate is a light green hygroscopic solid.[6] Both are soluble in water.[10] Thulium(III) chloride has a monoclinic crystal structure with the space group C2/m (No. 12) corresponding to that of aluminum(III) chloride.[10][8]
Thulium(III) chloride reacts with strong bases to make thulium(III) oxide.
References
- ↑ 1.0 1.1 Spencer, James F. (1919). "The Metals of the Rare Earths". New York: Longmans, Green, and Co. pp. 152. https://archive.org/details/metalsrareearth00spengoog.
- ↑ "Chemistry: Periodic Table: Thulium: compound data (thulium (III) chloride)". WebElements. http://202.114.88.54/g/web18/wangluo/webelements/webelements/compounds/text/tm/cl3tm1-13537183.html.
- ↑ Perry, Dale L.; Phillips, Sidney L. (1995). Handbook of Inorganic Compounds. CRC Press. pp. 512. ISBN 0-8493-8671-3. https://books.google.com/books?id=kTnxSi2B2FcC&pg=PT1017. Retrieved 2008-06-27.
- ↑ "Thulium trichloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/61643#section=Safety-and-Hazards.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN:0-19-855370-6
- ↑ 6.0 6.1 Sigma-Aldrich Co., Thulium(III) chloride hexahydrate, 99.99% trace metals basis.
- ↑ Bai, Lijie; Jiang, Wenya; Gao, Chunxiao; Zhong, Shuxian; Zhao, Leihong; Li, Zhengquan; Bai, Song (2016-11-17). "Facet engineered interface design of NaYF4:Yb,Tm upconversion nanocrystals on BiOCl nanoplates for enhanced near-infrared photocatalysis" (in en). Nanoscale 8 (45): 19014–19024. doi:10.1039/C6NR05720A. ISSN 2040-3372. PMID 27808315. https://pubs.rsc.org/en/content/articlelanding/2016/nr/c6nr05720a.
- ↑ 8.0 8.1 8.2 Handbuch der präparativen anorganischen Chemie. 2 (3., umgearb. Aufl ed.). Stuttgart: Enke. 1978. ISBN 978-3-432-87813-3.
- ↑ Webelements: Thulium
- ↑ 10.0 10.1 Ans, Jean d'; Lax, Ellen (1998) (in de). Taschenbuch für Chemiker und Physiker. Springer. ISBN 978-3-540-60035-0. https://books.google.com/books?id=ssy59etLaksC&pg=PA780.
 |

