Chemistry:Tungsten(III) chloride
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Cl18W6 | |
| Molar mass | 1741.14 g·mol−1 |
| Appearance | yellow brown solid |
| Density | 5.44 g·cm−3 |
| Melting point | 550[1] °C (1,022 °F; 823 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
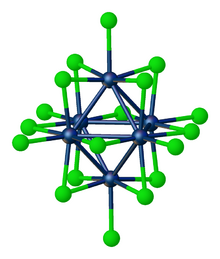
Tungsten(III) chloride is the inorganic compound with the formula W6Cl18. It is a cluster compound. It is a brown solid, obtainable by chlorination of tungsten(II) chloride.[2] Featuring twelve doubly bridging chloride ligands, the cluster adopts a structure related to the corresponding chlorides of niobium and tantalum. In contrast, W6Cl12 features eight triply bridging chlorides.
A related mixed valence W(III)-W(IV) chloride is prepared by reduction of the hexachloride with bismuth:[3]
- 9 WCl6 + 8 Bi → 3 W3Cl10 + 8 BiCl3
References
- ↑ Eliseev, S. S.; Synthesis and physicochemical properties of tungsten trichloride. Izvestiya Akademii Nauk SSSR, Neorganicheskie Materialy 1983, V19(7), P1182-5 CAPLUS
- ↑ Yue‐Qing Zheng; Ekaterina Jonas; Jürgen Nuss; Hans Georg von Schnering (1999). "The DMSO Solvated octahedro‐[W6iCl12aCl6 Cluster Molecule". Z. Anorg. Allg. Chem. 624: 1400–1404. doi:10.1002/(SICI)1521-3749(199809)624:9<1400::AID-ZAAC1400>3.0.CO;2-0.
- ↑ Thurston, J. H.; Kolesnichenko, V.; Messerle, L. (2014). "Trinuclear Tungsten Halide Clusters". Inorganic Syntheses: Volume 36. 36. 24–30. doi:10.1002/9781118744994.ch5. ISBN 978-1-118-74499-4.
 |

