Chemistry:Nitroso
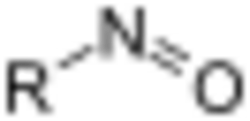
In organic chemistry, nitroso refers to a functional group in which the nitric oxide (–N=O) group is attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds (e.g., nitrosoalkanes; R–N=O), S-nitroso compounds (nitrosothiols; RS–N=O), N-nitroso compounds (e.g., nitrosamines, RN(–R’)–N=O), and O-nitroso compounds (alkyl nitrites; RO–N=O).
Synthesis
Nitroso compounds can be prepared by the reduction of nitro compounds[1] or by the oxidation of hydroxylamines.[2] Ortho-nitrosophenols may be produced by the Baudisch reaction. In the Fischer–Hepp rearrangement aromatic 4-nitrosoanilines are prepared from the corresponding nitrosamines.
Properties
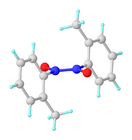
Nitrosoarenes typically participate in a monomer–dimer equilibrium. The dimers, which are often pale yellow, are often favored in the solid state, whereas the deep-green monomers are favored in dilute solution or at higher temperatures. They exist as cis and trans isomers.[4]
When stored in protic media, primary and secondary nitrosoalkanes isomerize to oximes.[5]
Due to the stability of the nitric oxide free radical, nitroso organyls tend to have very low C–N bond dissociation energies: nitrosoalkanes have BDEs on the order of 30–40 kcal/mol (130–170 kJ/mol), while nitrosoarenes have BDEs on the order of 50–60 kcal/mol (210–250 kJ/mol). As a consequence, they are generally heat- and light-sensitive. Compounds containing O–(NO) or N–(NO) bonds generally have even lower bond dissociation energies. For instance, N-nitrosodiphenylamine, Ph2N–N=O, has a N–N bond dissociation energy of only 23 kcal/mol (96 kJ/mol).[6] Organonitroso compounds serve as a ligands giving transition metal nitroso complexes.[7]
Reactions
Many reaction exists which make use of an intermediate nitroso compound, such as the Barton reaction and Davis–Beirut reaction, as well as in the synthesis of indoles, for example: Baeyer–Emmerling indole synthesis, Bartoli indole synthesis. In the Saville reaction, mercury is used to replace a nitrosyl from a thiol group.
C-nitroso compounds are used in organic synthesis as synthons in some well-documented chemical reactions such as hetero Diels-Alder (HDA), nitroso-ene and nitroso-aldol reactions.[8]
Nitrosation vs. nitrosylation
Nitrite can enter two kinds of reaction, depending on the physico-chemical environment.
- Nitrosylation is adding a nitrosyl ion NO−
to a metal (e.g. iron) or a thiol, leading to nitrosyl iron Fe–NO (e.g., in nitrosylated heme = nitrosylheme) or S-nitrosothiols (RSNOs). - Nitrosation is adding a nitrosonium ion NO+
to an amine –NH
2 leading to a nitrosamine. This conversion occurs at acidic pH, particularly in the stomach, as shown in the equation for the formation of N-phenylnitrosamine:- [math]\ce{ NO2- + H+ <=> HONO }[/math]
- [math]\ce{ HONO + H+ <=> H2O + NO+ }[/math]
- [math]\ce{ C6H5NH2 + NO+ -> C6H5N(H)NO + H+ }[/math]
Many primary alkyl N-nitroso compounds, such as CH
3N(H)NO, tend to be unstable with respect to hydrolysis to the alcohol. Those derived from secondary amines (e.g., (CH
3)
2NNO derived from dimethylamine) are more robust. It is these N-nitrosamines that are carcinogens in rodents.
Nitrosyl in inorganic chemistry
Nitrosyls are non-organic compounds containing the NO group, for example directly bound to the metal via the N atom, giving a metal–NO moiety. Alternatively, a nonmetal example is the common reagent nitrosyl chloride (Cl–N=O). Nitric oxide is a stable radical, having an unpaired electron. Reduction of nitric oxide gives the nitrosyl anion, NO−
:
- [math]\ce{ NO + e- -> NO- }[/math]
Oxidation of NO yields the nitrosonium cation, NO+
:
- [math]\ce{ NO -> NO+ + e- }[/math]
Nitric oxide can serve as a ligand forming metal nitrosyl complexes or just metal nitrosyls. These complexes can be viewed as adducts of NO+
, NO−
, or some intermediate case.
In human health
Nitrosamine formation during digestion
See also
- Nitrosamine, the functional group with the NO attached to an amine, such as R2N–NO
- Nitrosobenzene
- Nitric oxide
- Nitroxyl
References
- ↑ G. H. Coleman; C. M. McCloskey; F. A. Stuart (1945). "Nitrosobenzene". Org. Synth. 25: 80. doi:10.15227/orgsyn.025.0080.
- ↑ Calder, A.; Forrester, A. R.; Hepburn, S. P.. "2-Methyl-2-nitrosopropane and Its Dimer". Organic Syntheses 52: 77. http://www.orgsyn.org/demo.aspx?prep=cv6p0803.; Collective Volume, 6, pp. 803
- ↑ E.Bosch (2014). "Structural Analysis of Methyl-Substituted Nitrosobenzenes and Nitrosoanisoles". J. Chem. Cryst. 98 (2): 44. doi:10.1007/s10870-013-0489-8.
- ↑ Beaudoin, D.; Wuest, J. D. (2016). "Dimerization of Aromatic C-Nitroso Compounds". Chemical Reviews 116 (1): 258–286. doi:10.1021/cr500520s. PMID 26730505.
- ↑ Kirby, G. W. (1977). "Electrophilic C-nitroso-compounds". Chemical Society Reviews 6: 2. doi:10.1039/CS9770600001none (Tilden lecture).
- ↑ Luo, Yu-Ran (2007). Comprehensive Handbook of Chemical Bond Energies. Boca Raton, FL: Taylor and Francis. ISBN 9781420007282.
- ↑ Lee, Jonghyuk; Chen, Li; West, Ann H.; Richter-Addo, George B. (2002). "Interactions of Organic Nitroso Compounds with Metals". Chemical Reviews 102 (4): 1019–1066. doi:10.1021/cr0000731. PMID 11942786.
- ↑ Bianchi, P.; Monbaliu, J. C. M. (2022). "Three decades of unveiling the complex chemistry of C-nitroso species with computational chemistry". Organic Chemistry Frontiers 9: 223–264. doi:10.1039/d1qo01415c.
 |


