Chemistry:Rivastigmine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Exelon, Prometax, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602009 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, transdermal patch |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60 to 72% |
| Protein binding | 40% |
| Metabolism | Liver, via pseudocholinesterase |
| Elimination half-life | 1.5 hours |
| Excretion | 97% in urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
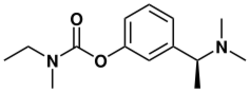
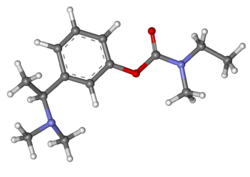
| Formula | C14H22N2O2 |
| Molar mass | 250.342 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rivastigmine (sold under the trade name Exelon among others) is a cholinesterase inhibitor used for the treatment of mild to moderate Alzheimer's disease.[1] The drug can be administered orally or via a transdermal patch; the latter form reduces the prevalence of side effects,[2] which typically include nausea and vomiting.[3]
The drug is eliminated through the urine, and appears to have relatively few drug-drug interactions.[3]
It was patented in 1985 and came into medical use in 1997.[4]
Medical uses
Rivastigmine capsules, liquid solution and patches are used for the treatment of mild to moderate dementia of the Alzheimer's type, and in the UK for mild to moderate Parkinson's disease dementia.[5]
Rivastigmine has demonstrated treatment effects on the cognitive (thinking and memory), functional (activities of daily living) and behavioural problems commonly associated with Alzheimer's.[6][7][8][9]
Efficacy
In people with either type of dementia, rivastigmine has been shown to provide meaningful symptomatic effects that may allow patients to remain independent and ‘be themselves’ for longer. In particular, it appears to show marked treatment effects in patients showing a more aggressive course of disease, such as those with younger onset ages, poor nutritional status, or those experiencing symptoms such as delusions or hallucinations.[10] For example, the presence of hallucinations appears to be a predictor of especially strong responses to rivastigmine, both in Alzheimer's and Parkinson's patients.[11][12] These effects might reflect the additional inhibition of butyrylcholinesterase, which is implicated in symptom progression and might provide added benefits over acetylcholinesterase-selective drugs in some patients.[10][11] Multiple-infarct dementia patients may show slight improvement in executive functions and behaviour. No firm evidence supports usage in schizophrenia patients.
Its efficacy is similar to donepezil and tacrine. Doses below 6 mg/d may be ineffective. The effects of this kind of drug in different kinds of dementia (including Alzheimer's dementia) are modest, and it is still unclear which AChE (BChE) esterase inhibitor is better in Parkinson's dementia, though rivastigmine is well-studied.
Side effects
Side effects may include nausea and vomiting, decreased appetite and weight loss.[3]
The strong potency of rivastigmine, provided by its dual inhibitory mechanism, has been postulated to lead to more nausea and vomiting during the titration phase of oral rivastigmine treatment.[3]
In a large clinical trial of the rivastigmine patch in 1,195 patients with Alzheimer's disease, the target dose of 9.5 mg/24-hour patch provided similar clinical effects (e.g. memory and thinking, activities of daily living, concentration) as the highest doses of rivastigmine capsules, but with one-third fewer reports of nausea and vomiting.[2]
Usage of rivastigmine was associated with a higher frequency of reports of death as an adverse event in the Food and Drug Administration Adverse Event Reporting System database compared to the other acetylcholinesterase inhibiting drugs donepezil and galantamine; this increase could be related to improper application of the transdermal patch, or because rivastigmine is more often used during advanced illness.[13]
Rivastigmine can increase gastric acid; it is discontinued if there are signs of gastrointestinal bleeding, particularly in individuals using nonsteroidal anti-inflammatory drugs (NSAIDs) or who have a history of peptic ulcer disease.[14]
Administration
Rivastigmine tartrate is a white to off-white, fine crystalline powder that is both lipophilic (soluble in fats) and hydrophilic (soluble in water). It comes in a variety of administrations including a capsule, solution and a transdermal patch. Like other cholinesterase inhibitors, it requires doses to be increased gradually over several weeks; this is usually referred to as the titration phase.[3]
Pharmacology
Pharmacodynamics
Rivastigmine, a cholinesterase inhibitor, inhibits both butyrylcholinesterase and acetylcholinesterase (unlike donepezil, which selectively inhibits acetylcholinesterase). It is thought to work by inhibiting these cholinesterase enzymes, which would otherwise break down the brain neurotransmitter acetylcholine.[15]
Pharmacokinetics
When given orally, rivastigmine is well absorbed, with a bioavailability of about 40% in the 3-mg dose. Pharmacokinetics are linear up to 3 mg BID, but nonlinear at higher doses. Elimination is through the urine. Peak plasma concentrations are seen in about one hour, with peak cerebrospinal fluid concentrations at 1.4–3.8 hours. When given by once-daily transdermal patch, the pharmacokinetic profile of rivastigmine is much smoother, compared with capsules, with lower peak plasma concentrations and reduced fluctuations.[16] The 9.5 mg/24 h rivastigmine patch provides comparable exposure to 12 mg/day capsules (the highest recommended oral dose).[16]
The compound does cross the blood–brain barrier. Plasma protein binding is 40%.[17] The major route of metabolism is by its target enzymes via cholinesterase-mediated hydrolysis. Elimination bypasses the hepatic system, so hepatic cytochrome P450 (CYP) isoenzymes are not involved.[18] The low potential for drug-drug interactions (which could lead to adverse effects) has been suggested as due to this pathway compared to the many common drugs that use the cytochrome P450 metabolic pathway.[3]
History
Rivastigmine was developed by Marta Weinstock-Rosin of the Department of Pharmacology at the Hebrew University of Jerusalem[19] and sold to Novartis by Yissum for commercial development. It is a semi-synthetic derivative of physostigmine.[20]
References
- ↑ "An update on the safety of current therapies for Alzheimer's disease: focus on rivastigmine". Therapeutic Advances in Drug Safety (SAGE Publications) 9 (3): 171–178. March 2018. doi:10.1177/2042098617750555. PMID 29492246.
- ↑ 2.0 2.1 "IDEAL: a 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease". Neurology 69 (4 Suppl 1): S14–S22. July 2007. doi:10.1212/01.wnl.0000281847.17519.e0. PMID 17646619.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "The tolerability and safety of cholinesterase inhibitors in the treatment of dementia". International Journal of Clinical Practice. Supplement (127): 45–63. June 2002. PMID 12139367.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 540. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA540.
- ↑ "Recommendations - Parkinson's disease in adults". National Institute for Health and Care Excellence. 2017-07-19. https://www.nice.org.uk/guidance/ng71/chapter/Recommendations.
- ↑ "A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease". International Journal of Geriatric Psychopharmacology 1 (2): 55–65. 1998. https://www.researchgate.net/publication/284814323.
- ↑ "Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial". BMJ 318 (7184): 633–638. March 1999. doi:10.1136/bmj.318.7184.633. PMID 10066203.
- ↑ "Effects of rivastigmine on behavioral and psychological symptoms of dementia in Alzheimer's disease". Clinical Therapeutics 26 (7): 980–990. July 2004. doi:10.1016/S0149-2918(04)90172-5. PMID 15336465.
- ↑ "Effects of two-year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer's disease". Behavioural Neurology 11 (4): 211–216. 1998. doi:10.1155/1999/168023. PMID 11568422.
- ↑ 10.0 10.1 "Aggressive course of disease in dementia". Alzheimer's & Dementia 2 (3): 210–217. July 2006. doi:10.1016/j.jalz.2006.03.002. PMID 19595889.
- ↑ 11.0 11.1 "Response to rivastigmine or donepezil in Alzheimer's patients with symptoms suggestive of concomitant Lewy body pathology". Current Medical Research and Opinion 22 (1): 49–59. January 2006. doi:10.1185/030079906X80279. PMID 16393430.
- ↑ "Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson's disease". Movement Disorders 21 (11): 1899–1907. November 2006. doi:10.1002/mds.21077. PMID 16960863.
- ↑ "Adverse Effects of Cholinesterase Inhibitors in Dementia, According to the Pharmacovigilance Databases of the United-States and Canada". PLOS ONE 10 (12): e0144337. 2015. doi:10.1371/journal.pone.0144337. PMID 26642212. Bibcode: 2015PLoSO..1044337A.
- ↑ "Drug monograph: Rivastigmine". Clinical Key database. Elsevier. p. 8. https://www.clinicalkey.com/#!/content/drug_monograph/6-s2.0-2294?p.
- ↑ "Cholinergic drugs in pharmacotherapy of Alzheimer's disease". Mini Reviews in Medicinal Chemistry 2 (1): 11–25. February 2002. doi:10.2174/1389557023406638. PMID 12369954.
- ↑ 16.0 16.1 "Pharmacokinetic rationale for the rivastigmine patch". Neurology 69 (4 Suppl 1): S10–S13. July 2007. doi:10.1212/01.wnl.0000281846.40390.50. PMID 17646618.
- ↑ "Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors". Clinical Pharmacokinetics 41 (10): 719–739. 2002. doi:10.2165/00003088-200241100-00003. PMID 12162759.
- ↑ "Rivastigmine, a new-generation cholinesterase inhibitor for the treatment of Alzheimer's disease". Pharmacotherapy 20 (1): 1–12. January 2000. doi:10.1592/phco.20.1.1.34664. PMID 10641971.
- ↑ "Exelon". Yissum Technology Transfer. http://www.yissum.co.il/success.php?cat=12&in=0.
- ↑ "Potential medicinal plants for CNS disorders: an overview". Phytotherapy Research 20 (12): 1023–1035. December 2006. doi:10.1002/ptr.1970. PMID 16909441.
External links
- "Rivastigmine". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/rivastigmine.
 |

