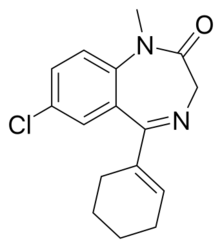
Chemistry:Tetrazepam
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3–26 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C16H17ClN2O |
| Molar mass | 288.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Tetrazepam[1] (is marketed under the following brand names, Clinoxan, Epsipam, Myolastan, Musaril, Relaxam and Spasmorelax) is a benzodiazepine derivative with anticonvulsant, anxiolytic, muscle relaxant and slightly hypnotic properties. It was formerly used mainly in Austria, France, Belgium, Germany and Spain to treat muscle spasm, anxiety disorders such as panic attacks, or more rarely to treat depression, premenstrual syndrome or agoraphobia. Tetrazepam has relatively little sedative effect at low doses while still producing useful muscle relaxation and anxiety relief. The Co-ordination Group for Mutual Recognition and Decentralised Procedures-Human (CMD(h)) endorsed the Pharmacovigilance Risk Assessment Committee (PRAC) recommendation to suspend the marketing authorisations of tetrazepam-containing medicines across the European Union (EU) in April 2013.[2] The European Commission has confirmed the suspension of the marketing authorisations for Tetrazepam in Europe because of cutaneous toxicity, effective from the 1 August 2013.[3]
Delayed type 4 allergic hypersensitivity reactions including maculopapular exanthema, erythematous rash, urticarial eruption, erythema multiforme, photodermatitis, eczema and Stevens–Johnson syndrome can occasionally occur as a result of tetrazepam exposure. These hypersensitivity reactions to tetrazepam share no cross-reactivity with other benzodiazepines.[4]
Indications
Tetrazepam is used therapeutically as a muscle relaxant.[5][6]
Availability
The indicated adult dose for muscle spasm is 25 mg to 150 mg per day, increased if necessary to a maximum of 300 mg per day, in divided doses. Tetrazepam is not generally recommended for use in children, except on the advice of a specialist.
Tetrazepam is only available in one strength and formulation, 50 mg tablets. The benzodiazepine equivalent of tetrazepam is approximately 100 mg of tetrazepam = 10 mg of diazepam.[7]
Adverse effects
Allergic reactions to tetrazepam occasionally occur involving the skin.[4]
Allergic reactions can develop to tetrazepam[8][9] and it is considered to be a potential allergen.[10][11] Drug rash and drug-induced eosinophilia with systemic symptoms is a known complication of tetrazepam exposure.[12][13] These hypersensitive allergic reactions can be of the delayed type.[14][15][16]
Toxic epidermal necrolysis has occurred from the use of tetrazepam[17][18] including at least one reported death.[19] Stevens–Johnson syndrome and erythema multiforme has been reported from use of tetrazepam. Cross-reactivity with other benzodiazepines does not typically occur in such patients.[20][21][22] Exanthema[23] and eczema may occur.[24] The lack of cross-reactivity with other benzodiazepines is believed to be due to the molecular structure of tetrazepam.[25][26] Photodermatitis[27] and phototoxicity have also been reported.[28] Occupational contact allergy can also develop from regularly handling tetrazepam.[29][30] Airborne contact dermatitis can also occur as an allergy which can develop from occupational exposure.[31]

Patch testing has been used successfully to demonstrate tetrazepam allergy.[32][33] Oral testing can also be used. Skin prick tests are not always accurate and may produce false negatives.[34]
Drowsiness is a common side effect of tetrazepam.[35] A reduction in muscle force can occur.[36] Myasthenia gravis, a condition characterised by severe muscle weakness is another potential adverse effect from tetrazepam.[37] Cardiovascular and respiratory adverse effects can occur with tetrazepam similar to other benzodiazepines.[26]
Tolerance, dependence and withdrawal
Prolonged use, as with all benzodiazepines, should be avoided, as tolerance occurs and there is a risk of benzodiazepine dependence and a benzodiazepine withdrawal syndrome after stopping or reducing dosage.[26]
Overdose
Tetrazepam, like other benzodiazepines is a drug which is very frequently present in cases of overdose. These overdoses are often mixed overdoses, i.e. a mixture of other benzodiazepines or other drug classes with tetrazepam.[38][39]
Contraindications and special caution
Benzodiazepines require special precaution if used in the elderly, during pregnancy, in children, alcohol or drug-dependent individuals and individuals with comorbid psychiatric disorders.[40]
Pharmacology
Tetrazepam is an unusual benzodiazepine in its molecular structure as it has cyclohexenyl group which has substituted the typical 5-phenyl moiety seen in other benzodiazepines.[41] Tetrazepam, is rapidly absorbed after oral administration, within 45 mins and reaches peak plasma levels in less than 2 hours. It is classed as an intermediate acting benzodiazepine with an elimination half-life of approximately 15 hours. It is primarily metabolised to the inactive metabolites 3-hydroxy-tetrazepam and nortetrazepam.[41][42] The pharmacological effects of tetrazepam are significantly less potent when compared against diazepam, in animal studies.[43] Tetrazepam is a benzodiazepine site agonist and binds unselectively to type 1 and type 2 benzodiazepine site types as well as to peripheral benzodiazepine receptors.[44] The muscle relaxant properties of tetrazepam are most likely due to a reduction of calcium influx.[45] Small amounts of diazepam as well as the active metabolites of diazepam are produced from metabolism of tetrazepam.[46][47] The metabolism of tetrazepam has led to false accusations of prisoners prescribed tetrazepam of taking illicit diazepam; this can lead to increased prison sentences for prisoners.[41]
Abuse
Tetrazepam as with other benzodiazepines is sometimes abused. It is sometimes abused to incapacitate a victim in order to carry out a drug-facilitated crime.[48] or abused in order to achieve a state of intoxication.[49] Tetrazepam's abuse for to carry out drug facilitated crimes may be less however, than other benzodiazepines due to its reduced hypnotic properties.[50]
See also
- Benzodiazepine
- Long-term effects of benzodiazepines
References
- ↑ NL Patent 6600095
- ↑ Recommendation to suspend tetrazepam-containing medicines endorsed by CMDh, European Medicines Agency, published 29 April 2013
- ↑ Ruhen der Zuhlassung aller Tetrazepam-haltiger Arzneimittel, Sanofi-Avensis Deutschland GmbH (German), published June 2013
- ↑ 4.0 4.1 "Acute generalized exanthematous pustulosis due to tetrazepam". Journal of Investigational Allergology & Clinical Immunology 18 (2): 119–22. 2008. PMID 18447141. http://www.jiaci.org/issues/vol18issue2/7.pdf.
- ↑ "Comparative study in mice of tetrazepam and other centrally active skeletal muscle relaxants". Archives Internationales de Pharmacodynamie et de Therapie 297: 272–85. January 1989. PMID 2567153.
- ↑ "Relaxant effect of tetrazepam on rat uterine smooth muscle: role of calcium movement". The Journal of Pharmacy and Pharmacology 48 (11): 1169–73. November 1996. doi:10.1111/j.2042-7158.1996.tb03915.x. PMID 8961167.
- ↑ "Benzodiazepine Names". non-benzodiazepines.org.uk. http://www.non-benzodiazepines.org.uk/benzodiazepine-names.html.
- ↑ "Tetrazepam allergy detected by patch test". Contact Dermatitis 22 (4): 246. April 1990. doi:10.1111/j.1600-0536.1990.tb01587.x. PMID 2140761.
- ↑ "Tetrazepam allergy once more detected by patch test". Contact Dermatitis 26 (4): 281. April 1992. doi:10.1111/j.1600-0536.1992.tb00259.x. PMID 1356710.
- ↑ "Tetrazepam: an allergen with several clinical expressions". Contact Dermatitis 33 (1): 63–5. July 1995. doi:10.1111/j.1600-0536.1995.tb00462.x. PMID 7493477.
- ↑ "Tetrazepam allergy". Allergy 54 (11): 1226–7. November 1999. doi:10.1034/j.1398-9995.1999.00362.x. PMID 10604563.
- ↑ "[Current differential diagnosis of hypereosinophilic syndrome]". Medicinski Pregled 60 (11–12): 581–6. November 2007. doi:10.2298/MPNS0712581D. PMID 18666600.
- ↑ "Probable drug rash with eosinophilia and systemic symptoms syndrome related to tetrazepam". Journal of the European Academy of Dermatology and Venereology 22 (7): 887–9. July 2008. doi:10.1111/j.1468-3083.2007.02490.x. PMID 18031497.
- ↑ "Delayed hypersensitivity to tetrazepam". Allergy 52 (11): 1146–7. November 1997. doi:10.1111/j.1398-9995.1997.tb00194.x. PMID 9404574.
- ↑ "Delayed cell-mediated hypersensitivity to tetrazepam". Contact Dermatitis 34 (2): 139. February 1996. doi:10.1111/j.1600-0536.1996.tb02147.x. PMID 8681544.
- ↑ "Cross-reactive type IV hypersensitivity reactions to benzodiazepines revealed by patch testing". Contact Dermatitis 33 (5): 356–7. November 1995. doi:10.1111/j.1600-0536.1995.tb02060.x. PMID 8565501.
- ↑ "Toxic epidermal necrolysis caused by tetrazepam". International Journal of Dermatology 45 (4): 480. April 2006. doi:10.1111/j.1365-4632.2006.02654.x. PMID 16650186.
- ↑ "Toxic epidermal necrolysis caused by tetrazepam". International Journal of Dermatology 45 (10): 1260–1. October 2006. doi:10.1111/j.1365-4632.2006.03061.x. PMID 17040464.
- ↑ "[Fatal toxic epidermal necrolysis associated with tetrazepam". Therapie 56 (2): 187–8. March 2001. PMID 11471372.
- ↑ "Tetrazepam drug sensitivity -- usefulness of the patch test". Contact Dermatitis 47 (3): 135–8. September 2002. doi:10.1034/j.1600-0536.2002.470302.x. PMID 12492544.
- ↑ "Stevens-Johnson syndrome from tetrazepam". Allergologia et Immunopathologia 26 (2): 55–7. 2 March 1998. PMID 9645262. http://www.elsevier.es/revistas/ctl_servlet?_f=7064&ip=90.216.52.234&articuloid=13011386&revistaid=105.
- ↑ "[Erythema multiforme by tetrazepam]". Medicina Clinica 115 (9): 359. September 2000. doi:10.1016/s0025-7753(00)71559-4. PMID 11093904.
- ↑ "[Tetrazepam (Myolastan)-induced exanthema: positive patch tests in 2 cases]". Annales de Dermatologie et de Vénéréologie 127 (12): 1094–6. December 2000. PMID 11173688.
- ↑ "Occupational airborne contact allergy to tetrazepam in a geriatric nurse". Journal of the German Society of Dermatology 7 (10): 896–8. October 2009. doi:10.1111/j.1610-0387.2009.07096.x. PMID 19453384.
- ↑ "No cross-reactions between tetrazepam and other benzodiazepines: a possible chemical explanation". Contact Dermatitis 61 (1): 53–6. July 2009. doi:10.1111/j.1600-0536.2009.01558.x. PMID 19659970.
- ↑ 26.0 26.1 26.2 "Erythema multiforme to tetrazepam". Journal of Investigational Allergology & Clinical Immunology 17 (3): 205–6. 2007. PMID 17583114. http://www.jiaci.org/issues/vol17issue03/14to17.pdf.
- ↑ "Photodermatitis from tetrazepam". Contact Dermatitis 39 (2): 84. August 1998. doi:10.1111/j.1600-0536.1998.tb05840.x. PMID 9746190.
- ↑ "Phototoxicity to tetrazepam - A new adverse reaction". Dermatology 197 (2): 193–4. 1998. PMID 9840980.
- ↑ "Occupational contact allergy to tetrazepam". Contact Dermatitis 44 (6): 372. June 2001. doi:10.1034/j.1600-0536.2001.440609-7.x. PMID 11417526.
- ↑ "Occupational airborne contact allergy to tetrazepam". Contact Dermatitis 49 (5): 260–1. November 2003. doi:10.1111/j.0105-1873.2003.0225c.x. PMID 14996051.
- ↑ "Occupational airborne contact dermatitis from sporadic exposure to tetrazepam during machine maintenance". Contact Dermatitis 52 (3): 173–4. March 2005. doi:10.1111/j.0105-1873.2005.0548o.x. PMID 15811045.
- ↑ "Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions". Contact Dermatitis 45 (6): 321–8. December 2001. doi:10.1034/j.1600-0536.2001.450601.x. PMID 11846746.
- ↑ "The usefulness of patch testing on the previously most severely affected site in a cutaneous adverse drug reaction to tetrazepam". Contact Dermatitis 44 (4): 259–60. April 2001. doi:10.1034/j.1600-0536.2001.440409-15.x. PMID 11336014.
- ↑ "Systemic dermatitis due to tetrazepam". Journal of Investigational Allergology & Clinical Immunology 18 (5): 404–6. 2008. PMID 18973107. http://www.jiaci.org/issues/vol18issue5/14.pdf.
- ↑ "[Medical treatment of spasticity]". Neuro-Chirurgie 49 (2–3 Pt 2): 247–55. May 2003. PMID 12746699.
- ↑ "[Clinical pilot study of the myogenic effects of flupirtine in comparison to tetrazepam and placebo]". Arzneimittel-Forschung 46 (3): 293–8. March 1996. PMID 8901152.
- ↑ "[Myasthenia gravis after tetrazepam treatment]". Anales de Medicina Interna 17 (12): 669. December 2000. doi:10.4321/s0212-71992000001200016. PMID 11213590.
- ↑ "[Determination of benzodiazepine derivatives mixture by high performance liquid chromatography]". Medicina 39 Suppl 2: 30–6. 2003. PMID 14617855.
- ↑ "[Analysis of benzodiazepine derivative mixture by gas-liquid chromatography"] (in lithuanian). Medicina 38 (3): 316–20. 2002. PMID 12474705. http://medicina.kmu.lt/0203/0203-12l.pdf.
- ↑ "Benzodiazepine dependence: focus on withdrawal syndrome". Annales Pharmaceutiques Françaises 67 (6): 408–13. November 2009. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ↑ 41.0 41.1 41.2 "Medicolegal aspects of tetrazepam metabolism". International Journal of Legal Medicine 121 (3): 169–74. May 2007. doi:10.1007/s00414-006-0118-6. PMID 17021899.
- ↑ "Biotransformation and pharmacokinetics of tetrazepam in man". Arzneimittel-Forschung 34 (6): 724–9. 1984. PMID 6148954.
- ↑ "Tetrazepam: a benzodiazepine which dissociates sedation from other benzodiazepine activities. I. Psychopharmacological profile in rodents". The Journal of Pharmacology and Experimental Therapeutics 245 (2): 692–8. May 1988. PMID 2896794.
- ↑ "Tetrazepam: a benzodiazepine which dissociates sedation from other benzodiazepine activities. II. In vitro and in vivo interactions with benzodiazepine binding sites". The Journal of Pharmacology and Experimental Therapeutics 245 (2): 699–705. May 1988. PMID 2896795.
- ↑ "Spasmolytic effects of tetrazepam on rat duodenum and guinea-pig ileum". Pharmacological Research 35 (5): 493–7. May 1997. doi:10.1006/phrs.1997.0173. PMID 9299217.
- ↑ "Metabolic studies of tetrazepam based on electrochemical simulation in comparison to in vivo and in vitro methods". Journal of Chromatography A 1216 (15): 3192–8. April 2009. doi:10.1016/j.chroma.2009.02.001. PMID 19233363.
- ↑ "Unraveling the metabolic transformation of tetrazepam to diazepam with mass spectrometric methods". Analytical and Bioanalytical Chemistry 392 (7–8): 1299–308. December 2008. doi:10.1007/s00216-008-2447-4. PMID 18949465.
- ↑ "Windows of detection of tetrazepam in urine, oral fluid, beard, and hair, with a special focus on drug-facilitated crimes". Therapeutic Drug Monitoring 27 (5): 565–70. October 2005. doi:10.1097/01.ftd.0000164610.14808.45. PMID 16175127.
- ↑ "Screening and confirmatory method for benzodiazepines and hypnotics in oral fluid by LC-MS/MS". Forensic Science International 150 (2–3): 213–20. June 2005. doi:10.1016/j.forsciint.2004.12.040. PMID 15944062.
- ↑ "Detection of diazepam in urine, hair and preserved oral fluid samples with LC-MS-MS after single and repeated administration of Myolastan and Valium". Analytical and Bioanalytical Chemistry 388 (7): 1545–56. August 2007. doi:10.1007/s00216-007-1297-9. PMID 17468852.
 |
