Chemistry:Curium(III) chloride
From HandWiki
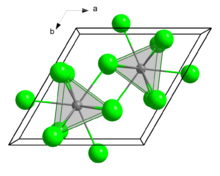 Crystal structure
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Cl3Cm | |
| Molar mass | 353 g·mol−1 |
| Melting point | 695 °C (1,283 °F; 968 K)[citation needed] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Curium(III) chloride is the chemical compound with the formula CmCl3.
Structure
Curium(III) chloride has a 9 coordinate tricapped trigonal prismatic geometry.[1]
Synthesis
- A reaction of hydrogen chloride gas with curium dioxide, curium(III) oxide, or curium(III) oxychloride at a temperature of 400-600 °C:
- CmOCl + 2HCl → CmCl
3 + H
2O
- CmOCl + 2HCl → CmCl
- Dissolution of metallic curium in dilute hydrochloric acid:[2]
- 2Cm + 6HCl → 2CmCl
3 + 3H
2
- 2Cm + 6HCl → 2CmCl
This method has a number of disadvantages associated with the ongoing processes of hydrolysis and hydration of the resulting compound in an aqueous solution. Thus, it is problematic to obtain a pure product using this reaction.
Preparation
Curium(III) chloride can be prepared by the reaction of curium nitride with cadmium chloride.[3]
References
- ↑ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Butterworth, UK. pp. 1270.
- ↑ Wallmann, J. C.; Fuger, J.; Peterson, J. R.; Green, J. L. (1 November 1967). "Crystal structure and lattice parameters of curium trichloride" (in en). Journal of Inorganic and Nuclear Chemistry 29 (11): 2745–2751. doi:10.1016/0022-1902(67)80013-7. ISSN 0022-1902. https://www.sciencedirect.com/science/article/abs/pii/0022190267800137. Retrieved 3 July 2023.
- ↑ Hayashi, Hirokazu; Takano, Masahide; Otobe, Haruyoshi; Koyama, Tadafumi (July 2013). "Syntheses and thermal analyses of curium trichloride". Journal of Radioanalytical and Nuclear Chemistry 297 (1): 139–144. doi:10.1007/s10967-012-2413-7.
 |

