Chemistry:Nitrogen difluoride
| |
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| NF 2 | |
| Molar mass | 52.004 g·mol−1 |
| Related compounds | |
Related nitrogen fluorides
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
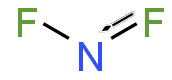
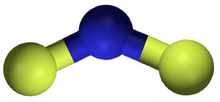
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF
2. This small molecule is in equilibrium with its dimer tetrafluorohydrazine.[2]
- N
2F
4 ⇌ 2 NF
2
As the temperature increases the proportion of NF
2 increases.[3]
The molecule is unusual in that it has an odd number of electrons, yet is stable enough to study experimentally.[4]
Properties
The energy needed to break the N–N bond in N
2F
4 is 20.8 kcal/mol (87 kJ/mol), with an entropy change of 38.6 eu.[5] For comparison, the dissociation energy of the N–N bond is 14.6 kcal/mol (61 kJ/mol) in N
2O
4, 10.2 kcal/mol (43 kJ/mol) in N
2O
2, and 60 kcal/mol (250 kJ/mol) in N
2H
4. The enthalpy of formation of N
2F
4 (ΔHf) is 8.227 kcal/mol (34.421 kJ/mol).[6]
At room temperature N
2F
4 is mostly associated with only 0.7% in the form of NF
2 at 5 mmHg (670 Pa) pressure. When the temperature rises to 225 °C, it mostly dissociates with 99% in the form of NF
2.[5]
In NF
2, the N–F bond length is 1.3494 Å and the angle subtended at F–N–F is 103.33°.[7]
In the infrared spectrum the N–F bond in NF
2 has a symmetrical stretching frequency of 1075 cm−1. This compares to 1115 cm−1 in NF, 1021 cm−1 in NF
3 and 998 cm−1 in N
2F
4.[5]
The microwave spectrum shows numerous lines due to spin transitions, with or without nuclear spin transitions. The lines form set of two triplets for antisymmetric singlet, or two triplets of triplets for symmetric triplet. Lines appear around 14–15, 24, 25, 26, 27, 28–29, 33, 60, 61, 62, and 65 GHz. The rotational constants for the NF
2 molecule are A = 70496 MHz, B = 11872.2 MHz, and C = 10136.5 MHz. The inertial defect Δ = 0.1204 mu·Å2. The centrifugal distortion constants are τaaaa = −7.75, τbbbb = −0.081, τaabb = 0.30, and τabab = −0.13.[7]
The dipole moment is 0.13 D (4.5×10−31 C·m).[7]
The ground electronic state of the molecule is 2B1.[7]
The gas is often contaminated with NO or N
2O.[5]
Use
Nitrogen difluoride is formed during the function of a xenon monofluoride excimer laser. Nitrogen trifluoride is the halide carrier gas, which releases fluoride ions when impacted by electrons:[1]
- NF
3 + e−
→ NF
2 + F−
The free fluoride ion goes on to react with xenon cations.[1]
Nitrogen difluoride can be consumed further to yield nitrogen monofluoride.[1]
- NF
2 + e−
→ NF + F−
References
- ↑ 1.0 1.1 1.2 1.3 Trainor, Daniel W. (February 1989). "Electron dissociative attachment to nitrogen difluoride radicals". The Journal of Physical Chemistry 93 (3): 1134–1136. doi:10.1021/j100340a022.
- ↑ Jäger, Susanne; von Jouanne, Jörn; Keller-Rudek, Hannelore; Koschel, Dieter; Kuhn, Peter; Merlet, Peter; Rupecht, Sigrid; Vanecek, Hans et al. (1986). Koschel, Dieter; Kuhn, Peter; Merlet, Peter et al.. eds. F Fluorine: Compounds with Oxygen and Nitrogen. Gmelin Handbook of Inorganic Chemistry. 4. Berlin: Springer. p. 162. doi:10.1007/978-3-662-06339-2. ISBN 978-3-662-06341-5. https://books.google.com/books?id=rpfsCAAAQBAJ&pg=PA162. Retrieved 29 August 2015.
- ↑ Johnson, Frederic A.; Colburn, Charles B. (July 1961). "The Tetrafluorohydrazine-Difluoroamino Radical Equilibrium". Journal of the American Chemical Society 83 (14): 3043–3047. doi:10.1021/ja01475a018.
- ↑ Brown, R. D.; Burden, F. R.; Hart, B. T.; Williams, G. R. (1973). "The electronic structure of the NF2 radical". Theoretica Chimica Acta 28 (4): 339–353. doi:10.1007/BF00529015.
- ↑ 5.0 5.1 5.2 5.3 Bohn, Robert K.; Bauer, Simon Harvey (February 1967). "An electron diffraction study of the structures of NF2 and N2F4". Inorganic Chemistry 6 (2): 304–309. doi:10.1021/ic50048a024. molecule dimensions and angles
- ↑ "Nitrogen difluoride NF2(g)". http://www.chem.msu.su/rus/handbook/ivtan/100.html.
- ↑ 7.0 7.1 7.2 7.3 Brown, R.D.; Burden, F.R.; Godfrey, P.D.; Gillard, I.R. (August 1974). "Microwave spectrum of NF2". Journal of Molecular Spectroscopy 52 (2): 301–321. doi:10.1016/0022-2852(74)90121-0. Bibcode: 1974JMoSp..52..301B.
Extra reading
- Goodfriend, P.L.; Woods, H.P. (January 1964). "The absorption spectrum of NF2". Journal of Molecular Spectroscopy 13 (1–4): 63–66. doi:10.1016/0022-2852(64)90055-4. Bibcode: 1964JMoSp..13...63G.
- Jacox, Marilyn E.; Milligan, Dolphus E.; Guillory, William A.; Smith, Jerry J. (August 1974). "Matrix-isolation study of the vacuum-ultraviolet photolysis of NF3". Journal of Molecular Spectroscopy 52 (2): 322–327. doi:10.1016/0022-2852(74)90122-2. Bibcode: 1974JMoSp..52..322J.
- Heidner, R. F.; Helvajian, Henry; Koffend, J. Brooke (August 1987). "Tunable UV laser photolysis of NF2: Quantum yield for NF(a1Δ) production". The Journal of Chemical Physics 87 (3): 1520–1524. doi:10.1063/1.453262. Bibcode: 1987JChPh..87.1520H. https://apps.dtic.mil/sti/pdfs/ADA194960.pdf.
- Papakondylis, Aristotle; Mavridis, Aristides (December 1993). "Electronic and geometrical structure of the NF2 radical". Chemical Physics Letters 216 (1–2): 167–172. doi:10.1016/0009-2614(93)E1254-E. Bibcode: 1993CPL...216..167P. http://jupiter.chem.uoa.gr/pchem/pubs/pdf/CPL_216(1994)167.pdf.
- Cai, Z.-L.; Sha, G.-H.; Zhang, C.-H.; Huang, M.-B. (March 1991). "Ab initio study of low-lying electronic states of the NF2 radical". Chemical Physics Letters 178 (2–3): 273–278. doi:10.1016/0009-2614(91)87068-M. Bibcode: 1991CPL...178..273C.
 |
