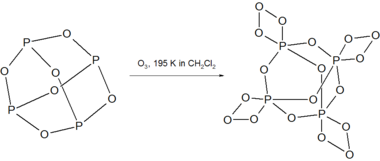Chemistry:Phosphorus trioxide
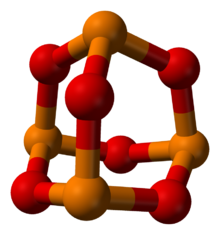 Phosphorus in orange, oxygen in red
| |
| |
| Names | |
|---|---|
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 26856 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| P4O6 | |
| Molar mass | 219.88 g mol−1 |
| Appearance | colourless monoclinic crystals or liquid |
| Density | 2.135 g/cm3 |
| Melting point | 23.8 °C (74.8 °F; 296.9 K) |
| Boiling point | 173.1 °C (343.6 °F; 446.2 K) |
| reacts | |
| Acidity (pKa) | 9.4 |
| Structure | |
| See Text | |
| 0 | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
Phosphorus trisulfide |
Other cations
|
Dinitrogen trioxide Arsenic trioxide Antimony trioxide |
Related compounds
|
Phosphorus pentoxide Phosphorous acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today. This colorless solid is structurally related to adamantane. It is formally the anhydride of phosphorous acid, H3PO3, but cannot be obtained by the dehydration of the acid. A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odor.[1]
Preparation
It is obtained by the combustion of phosphorus in a limited supply of air at low temperatures.
- P4 + 3 O2 → P4O6
By-products include red phosphorus suboxide.[1]
Chemical properties
Phosphorus trioxide reacts with water to form phosphorous acid, reflecting the fact that it is the anhydride of that acid.[2]
- P4O6 + 6 H2O → 4 H3PO3
It reacts with hydrogen chloride to form H3PO3 and phosphorus trichloride.
- P4O6 + 6 HCl → 2 H3PO3 + 2 PCl3
With chlorine or bromine it forms the corresponding phosphoryl halide, and it reacts with iodine in a sealed tube to form diphosphorus tetraiodide.[1]
P4O6 reacts with ozone at 195 K to give the unstable compound P4O18.[3]
P4O18 decomposes above 238 K in solution with the release of O2 gas. Decomposition of dry P4O18 is explosive.
In a disproportionation reaction, P4O6 is converted into the mixed P(III)P(V) species P4O8 when heated in a sealed tube at 710 K, with the side product being red phosphorus.[3]
As a ligand
P4O6 is a ligand for transition metals, comparable to phosphite. An illustrative complex is P4O6·Fe(CO)4.[4] With BH3, a dimeric adduct is produced:[3]
References
- ↑ 1.0 1.1 1.2 A. F. Holleman; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Boston: Academic Press. ISBN 0-12-352651-5.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 3.0 3.1 3.2 .Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 473. ISBN 978-0-13-175553-6.
- ↑ M. Jansen; J. Clade (November 1996). "Tetracarbonyl(tetraphosphorus hexoxide)iron". Acta Crystallogr. C 52 (11): 2650–2652. doi:10.1107/S0108270196004398.
 |