Physics:Spin
Spin is an intrinsic form of angular momentum carried by elementary particles, and thus by composite particles such as hadrons, atomic nuclei, and atoms.[1][2]:{{{1}}} The spinning electron was originally proposed as a small rigid particle rotating about an axis,[3] as ordinary use of the word may suggest. Instead, as described by Ohanian, "spin may be regarded as an angular momentum generated by a circulating flow of charge in the wave field of the electron".[3]
The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum.[4] The relativistic spin–statistics theorem connects electron spin quantization to the Pauli exclusion principle: observations of exclusion imply spin and observations of spin imply exclusion.
Spin is described mathematically as a vector for some particles such as photons, and as spinors and bispinors for other particles such as electrons. Spinors and bispinors behave similarly to vectors: they have definite magnitudes and change under rotations; however, they use an unconventional "direction". All elementary particles of a given kind have the same magnitude of spin angular momentum, though its direction may change. These are indicated by assigning the particle a spin quantum number.[2]:{{{1}}}
The SI units of spin are the same as classical angular momentum (i.e., N·m·s, J·s, or kg·m2·s−1). In quantum mechanics, angular momentum and spin angular momentum take discrete values proportional to the Planck constant. In practice, spin is usually given as a dimensionless spin quantum number by dividing the spin angular momentum by the reduced Planck constant ħ. Often, the "spin quantum number" is simply called "spin".
Relation to classical rotation
The very earliest models for electron spin imagined a rotating charged mass, but this model fails when examined in detail: the required space distribution does not match limits on the electron radius: the required rotation speed exceeds the speed of light. In the Standard Model, the fundamental particles are all considered "point-like": they have their effects through the field that surrounds them.[5] Any model for spin based on mass rotation would need to be consistent with that model.
The classical analog for quantum spin is a circulation of energy or momentum-density in the particle wave field: "spin is essentially a wave property".[3] This same concept of spin can be applied to gravity waves in water: "spin is generated by subwavelength circular motion of water particles".[6]
Unlike classical wavefield circulation which allows continuous values of angular momentum, quantum wavefields allow only discrete values.[3] Consequently energy transfer to or from spins states always occurs in fixed quantum steps. Only a few steps are allowed: for many qualitative purposes the complexity of the spin quantum wavefields can be ignored and the system properties can be discussed in terms of "integer" or "half-integer" spin models as discussed in quantum numbers below.
Photon spin is the quantum-mechanical description of light polarization, where spin +1 and spin −1 represent two opposite directions of circular polarization. Thus, light of a defined circular polarization consists of photons with the same spin, either all +1 or all −1. Spin represents polarization for other vector bosons as well.
Relation to orbital angular momentum
As the name suggests, spin was originally conceived as the rotation of a particle around some axis. Historically orbital angular momentum related to particle orbits.[7]:131 While the names based on mechanical models have survived, the physical explanation has not. Quantization fundamentally alters the character of both spin and orbital angular momentum.
Since elementary particles are point-like, self-rotation is not well-defined for them. However, spin implies that the phase of the particle depends on the angle as [math]\displaystyle{ e^{i S \theta} }[/math], for rotation of angle θ around the axis parallel to the spin S. This is equivalent to the quantum-mechanical interpretation of momentum as phase dependence in the position, and of orbital angular momentum as phase dependence in the angular position.
For fermions, the picture is less clear. Angular velocity is equal by the Ehrenfest theorem to the derivative of the Hamiltonian to its conjugate momentum, which is the total angular momentum operator J = L + S. Therefore, if the Hamiltonian H is dependent upon the spin S, dH/dS is non-zero, and the spin causes angular velocity, and hence actual rotation, i.e. a change in the phase-angle relation over time. However, whether this holds for free electron is ambiguous, since for an electron, S2 is constant, and therefore it is a matter of interpretation whether the Hamiltonian includes such a term. Nevertheless, spin appears in the Dirac equation, and thus the relativistic Hamiltonian of the electron, treated as a Dirac field, can be interpreted as including a dependence in the spin S.[8] Under this interpretation, free electrons also self-rotate, with the zitterbewegung effect understood as this rotation.
Quantum number
Spin obeys the mathematical laws of angular momentum quantization. The specific properties of spin angular momenta include:
- Spin quantum numbers may take half-integer values.
- Although the direction of its spin can be changed, the magnitude of the spin of an elementary particle cannot be changed.
- The spin of a charged particle is associated with a magnetic dipole moment with a g-factor that differs from 1. (In the classical context, this would imply the internal charge and mass distributions differing for a rotating object.[9])
The conventional definition of the spin quantum number is s = n/2, where n can be any non-negative integer. Hence the allowed values of s are 0, 1/2, 1, 3/2, 2, etc. The value of s for an elementary particle depends only on the type of particle and cannot be altered in any known way (in contrast to the spin direction described below). The spin angular momentum S of any physical system is quantized. The allowed values of S are [math]\displaystyle{ S = \hbar \, \sqrt{s(s + 1)} = \frac{h}{2\pi} \, \sqrt{\frac{n}{2}\frac{(n + 2)}{2}} = \frac{h}{4\pi} \, \sqrt{n(n + 2)}, }[/math] where h is the Planck constant, and [math]\displaystyle{ \hbar = \frac{h}{2\pi} }[/math] is the reduced Planck constant. In contrast, orbital angular momentum can only take on integer values of s; i.e., even-numbered values of n.
Fermions and bosons
Those particles with half-integer spins, such as 1/2, 3/2, 5/2, are known as fermions, while those particles with integer spins, such as 0, 1, 2, are known as bosons. The two families of particles obey different rules and broadly have different roles in the world around us. A key distinction between the two families is that fermions obey the Pauli exclusion principle: that is, there cannot be two identical fermions simultaneously having the same quantum numbers (meaning, roughly, having the same position, velocity and spin direction). Fermions obey the rules of Fermi–Dirac statistics. In contrast, bosons obey the rules of Bose–Einstein statistics and have no such restriction, so they may "bunch together" in identical states. Also, composite particles can have spins different from their component particles. For example, a helium-4 atom in the ground state has spin 0 and behaves like a boson, even though the quarks and electrons which make it up are all fermions.
This has some profound consequences:
- Quarks and leptons (including electrons and neutrinos), which make up what is classically known as matter, are all fermions with spin 1/2. The common idea that "matter takes up space" actually comes from the Pauli exclusion principle acting on these particles to prevent the fermions from being in the same quantum state. Further compaction would require electrons to occupy the same energy states, and therefore a kind of pressure (sometimes known as degeneracy pressure of electrons) acts to resist the fermions being overly close. Elementary fermions with other spins (3/2, 5/2, etc.) are not known to exist.
- Elementary particles which are thought of as carrying forces are all bosons with spin 1. They include the photon, which carries the electromagnetic force, the gluon (strong force), and the W and Z bosons (weak force). The ability of bosons to occupy the same quantum state is used in the laser, which aligns many photons having the same quantum number (the same direction and frequency), superfluid liquid helium resulting from helium-4 atoms being bosons, and superconductivity, where pairs of electrons (which individually are fermions) act as single composite bosons. Elementary bosons with other spins (0, 2, 3, etc.) were not historically known to exist, although they have received considerable theoretical treatment and are well established within their respective mainstream theories. In particular, theoreticians have proposed the graviton (predicted to exist by some quantum gravity theories) with spin 2, and the Higgs boson (explaining electroweak symmetry breaking) with spin 0. Since 2013, the Higgs boson with spin 0 has been considered proven to exist.[10] It is the first scalar elementary particle (spin 0) known to exist in nature.
- Atomic nuclei have nuclear spin which may be either half-integer or integer, so that the nuclei may be either fermions or bosons.
Spin–statistics theorem
The spin–statistics theorem splits particles into two groups: bosons and fermions, where bosons obey Bose–Einstein statistics, and fermions obey Fermi–Dirac statistics (and therefore the Pauli exclusion principle). Specifically, the theorem requires that particles with half-integer spins obey the Pauli exclusion principle while particles with integer spin do not. As an example, electrons have half-integer spin and are fermions that obey the Pauli exclusion principle, while photons have integer spin and do not. The theorem was derived by Wolfgang Pauli in 1940; it relies on both quantum mechanics and the theory of special relativity. Pauli described this connection between spin and statistics as "one of the most important applications of the special relativity theory".[11]
Magnetic moments
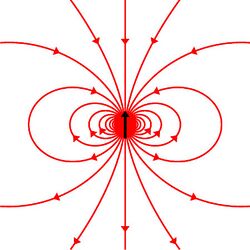
Particles with spin can possess a magnetic dipole moment, just like a rotating electrically charged body in classical electrodynamics. These magnetic moments can be experimentally observed in several ways, e.g. by the deflection of particles by inhomogeneous magnetic fields in a Stern–Gerlach experiment, or by measuring the magnetic fields generated by the particles themselves.
The intrinsic magnetic moment μ of a spin-1/2 particle with charge q, mass m, and spin angular momentum S, is[12]
- [math]\displaystyle{ \boldsymbol{\mu} = \frac{g_\text{s} q}{2m} \mathbf{S}, }[/math]
where the dimensionless quantity gs is called the spin g-factor. For exclusively orbital rotations it would be 1 (assuming that the mass and the charge occupy spheres of equal radius).
The electron, being a charged elementary particle, possesses a nonzero magnetic moment. One of the triumphs of the theory of quantum electrodynamics is its accurate prediction of the electron g-factor, which has been experimentally determined to have the value −2.00231930436256(35), with the digits in parentheses denoting measurement uncertainty in the last two digits at one standard deviation.[13] The value of 2 arises from the Dirac equation, a fundamental equation connecting the electron's spin with its electromagnetic properties, and the deviation from −2 arises from the electron's interaction with the surrounding electromagnetic field, including its own field.[14]
Composite particles also possess magnetic moments associated with their spin. In particular, the neutron possesses a non-zero magnetic moment despite being electrically neutral. This fact was an early indication that the neutron is not an elementary particle. In fact, it is made up of quarks, which are electrically charged particles. The magnetic moment of the neutron comes from the spins of the individual quarks and their orbital motions.
Neutrinos are both elementary and electrically neutral. The minimally extended Standard Model that takes into account non-zero neutrino masses predicts neutrino magnetic moments of:[15][16][17]
- [math]\displaystyle{ \mu_\nu \approx 3 \times 10^{-19} \mu_\text{B} \frac{m_\nu c^2}{\text{eV}}, }[/math]
where the μν are the neutrino magnetic moments, mν are the neutrino masses, and μB is the Bohr magneton. New physics above the electroweak scale could, however, lead to significantly higher neutrino magnetic moments. It can be shown in a model-independent way that neutrino magnetic moments larger than about 10−14 μB are "unnatural" because they would also lead to large radiative contributions to the neutrino mass. Since the neutrino masses are known to be at most about 1 eV/c2, fine-tuning would be necessary in order to prevent large contributions to the neutrino mass via radiative corrections.[18] The measurement of neutrino magnetic moments is an active area of research. Experimental results have put the neutrino magnetic moment at less than 1.2×10−10 times the electron's magnetic moment.
On the other hand elementary particles with spin but without electric charge, such as a photon or a Z boson, do not have a magnetic moment.
Curie temperature and loss of alignment
In ordinary materials, the magnetic dipole moments of individual atoms produce magnetic fields that cancel one another, because each dipole points in a random direction, with the overall average being very near zero. Ferromagnetic materials below their Curie temperature, however, exhibit magnetic domains in which the atomic dipole moments spontaneously align locally, producing a macroscopic, non-zero magnetic field from the domain. These are the ordinary "magnets" with which we are all familiar.
In paramagnetic materials, the magnetic dipole moments of individual atoms will partially align with an externally applied magnetic field. In diamagnetic materials, on the other hand, the magnetic dipole moments of individual atoms align oppositely to any externally applied magnetic field, even if it requires energy to do so.
The study of the behavior of such "spin models" is a thriving area of research in condensed matter physics. For instance, the Ising model describes spins (dipoles) that have only two possible states, up and down, whereas in the Heisenberg model the spin vector is allowed to point in any direction. These models have many interesting properties, which have led to interesting results in the theory of phase transitions.
Direction
Spin projection quantum number and multiplicity
In classical mechanics, the angular momentum of a particle possesses not only a magnitude (how fast the body is rotating), but also a direction (either up or down on the axis of rotation of the particle). Quantum-mechanical spin also contains information about direction, but in a more subtle form. Quantum mechanics states that the component of angular momentum for a spin-s particle measured along any direction can only take on the values[19]
- [math]\displaystyle{ S_i = \hbar s_i, \quad s_i \in \{ -s, -(s - 1), \dots, s - 1, s \}, }[/math]
where Si is the spin component along the i-th axis (either x, y, or z), si is the spin projection quantum number along the i-th axis, and s is the principal spin quantum number (discussed in the previous section). Conventionally the direction chosen is the z axis:
- [math]\displaystyle{ S_z = \hbar s_z, \quad s_z \in \{ -s, -(s - 1), \dots, s - 1, s \}, }[/math]
where Sz is the spin component along the z axis, sz is the spin projection quantum number along the z axis.
One can see that there are 2s + 1 possible values of sz. The number "2s + 1" is the multiplicity of the spin system. For example, there are only two possible values for a spin-1/2 particle: sz = +1/2 and sz = −1/2. These correspond to quantum states in which the spin component is pointing in the +z or −z directions respectively, and are often referred to as "spin up" and "spin down". For a spin-3/2 particle, like a delta baryon, the possible values are +3/2, +1/2, −1/2, −3/2.
Vector
For a given quantum state, one could think of a spin vector [math]\displaystyle{ \lang S \rang }[/math] whose components are the expectation values of the spin components along each axis, i.e., [math]\displaystyle{ \lang S \rang = [\lang S_x \rang, \lang S_y \rang, \lang S_z \rang] }[/math]. This vector then would describe the "direction" in which the spin is pointing, corresponding to the classical concept of the axis of rotation. It turns out that the spin vector is not very useful in actual quantum-mechanical calculations, because it cannot be measured directly: sx, sy and sz cannot possess simultaneous definite values, because of a quantum uncertainty relation between them. However, for statistically large collections of particles that have been placed in the same pure quantum state, such as through the use of a Stern–Gerlach apparatus, the spin vector does have a well-defined experimental meaning: It specifies the direction in ordinary space in which a subsequent detector must be oriented in order to achieve the maximum possible probability (100%) of detecting every particle in the collection. For spin-1/2 particles, this probability drops off smoothly as the angle between the spin vector and the detector increases, until at an angle of 180°—that is, for detectors oriented in the opposite direction to the spin vector—the expectation of detecting particles from the collection reaches a minimum of 0%.
As a qualitative concept, the spin vector is often handy because it is easy to picture classically. For instance, quantum-mechanical spin can exhibit phenomena analogous to classical gyroscopic effects. For example, one can exert a kind of "torque" on an electron by putting it in a magnetic field (the field acts upon the electron's intrinsic magnetic dipole moment—see the following section). The result is that the spin vector undergoes precession, just like a classical gyroscope. This phenomenon is known as electron spin resonance (ESR). The equivalent behaviour of protons in atomic nuclei is used in nuclear magnetic resonance (NMR) spectroscopy and imaging.
Mathematically, quantum-mechanical spin states are described by vector-like objects known as spinors. There are subtle differences between the behavior of spinors and vectors under coordinate rotations. For example, rotating a spin-1/2 particle by 360° does not bring it back to the same quantum state, but to the state with the opposite quantum phase; this is detectable, in principle, with interference experiments. To return the particle to its exact original state, one needs a 720° rotation. (The plate trick and Möbius strip give non-quantum analogies.) A spin-zero particle can only have a single quantum state, even after torque is applied. Rotating a spin-2 particle 180° can bring it back to the same quantum state, and a spin-4 particle should be rotated 90° to bring it back to the same quantum state. The spin-2 particle can be analogous to a straight stick that looks the same even after it is rotated 180°, and a spin-0 particle can be imagined as sphere, which looks the same after whatever angle it is turned through.
Mathematical formulation
Operator
Spin obeys commutation relations[20] analogous to those of the orbital angular momentum: [math]\displaystyle{ \left[\hat S_j, \hat S_k\right] = i \hbar \varepsilon_{jkl} \hat S_l, }[/math] where εjkl is the Levi-Civita symbol. It follows (as with angular momentum) that the eigenvectors of [math]\displaystyle{ \hat S^2 }[/math] and [math]\displaystyle{ \hat S_z }[/math] (expressed as kets in the total S basis) are[2](p166) [math]\displaystyle{ \begin{align} \hat S^2 |s, m_s\rangle &= \hbar^2 s(s + 1) |s, m_s\rangle, \\ \hat S_z |s, m_s\rangle &= \hbar m_s |s, m_s\rangle. \end{align} }[/math]
The spin raising and lowering operators acting on these eigenvectors give [math]\displaystyle{ \hat S_\pm |s, m_s\rangle = \hbar \sqrt{s(s + 1) - m_s(m_s \pm 1)} |s, m_s \pm 1\rangle, }[/math] where [math]\displaystyle{ \hat S_\pm = \hat S_x \pm i \hat S_y }[/math].[2](p166)
But unlike orbital angular momentum, the eigenvectors are not spherical harmonics. They are not functions of θ and φ. There is also no reason to exclude half-integer values of s and ms.
All quantum-mechanical particles possess an intrinsic spin [math]\displaystyle{ s }[/math] (though this value may be equal to zero). The projection of the spin [math]\displaystyle{ s }[/math] on any axis is quantized in units of the reduced Planck constant, such that the state function of the particle is, say, not [math]\displaystyle{ \psi = \psi(\mathbf r) }[/math], but [math]\displaystyle{ \psi = \psi(\mathbf r,s_z) }[/math], where [math]\displaystyle{ s_z }[/math] can take only the values of the following discrete set: [math]\displaystyle{ s_z \in \{-s\hbar, -(s - 1)\hbar, \dots, +(s - 1)\hbar, +s\hbar\}. }[/math]
One distinguishes bosons (integer spin) and fermions (half-integer spin). The total angular momentum conserved in interaction processes is then the sum of the orbital angular momentum and the spin.
Pauli matrices
The quantum-mechanical operators associated with spin-1/2 observables are [math]\displaystyle{ \hat{\mathbf{S}} = \frac{\hbar}{2} \boldsymbol{\sigma}, }[/math] where in Cartesian components [math]\displaystyle{ S_x = \frac{\hbar}{2} \sigma_x, \quad S_y = \frac{\hbar}{2} \sigma_y, \quad S_z = \frac{\hbar}{2} \sigma_z. }[/math]
For the special case of spin-1/2 particles, σx, σy and σz are the three Pauli matrices: [math]\displaystyle{ \sigma_x = \begin{pmatrix} 0 & 1\\ 1 & 0 \end{pmatrix}, \quad \sigma_y = \begin{pmatrix} 0 & -i\\ i & 0 \end{pmatrix}, \quad \sigma_z = \begin{pmatrix} 1 & 0\\ 0 & -1 \end{pmatrix}. }[/math]
Pauli exclusion principle
The Pauli exclusion principle states that the wavefunction [math]\displaystyle{ \psi(\mathbf r_1, \sigma_1, \dots, \mathbf r_N, \sigma_N) }[/math] for a system of N identical particles having spin s must change upon interchanges of any two of the N particles as [math]\displaystyle{ \psi(\dots, \mathbf r_i, \sigma_i, \dots, \mathbf r_j, \sigma_j, \dots ) = (-1)^{2s} \psi(\dots, \mathbf r_j, \sigma_j, \dots, \mathbf r_i, \sigma_i, \dots). }[/math]
Thus, for bosons the prefactor (−1)2s will reduce to +1, for fermions to −1. This permutation postulate for N-particle state functions has most important consequences in daily life, e.g. the periodic table of the chemical elements.
Rotations
As described above, quantum mechanics states that components of angular momentum measured along any direction can only take a number of discrete values. The most convenient quantum-mechanical description of particle's spin is therefore with a set of complex numbers corresponding to amplitudes of finding a given value of projection of its intrinsic angular momentum on a given axis. For instance, for a spin-1/2 particle, we would need two numbers a±1/2, giving amplitudes of finding it with projection of angular momentum equal to +ħ/2 and −ħ/2, satisfying the requirement [math]\displaystyle{ |a_{+1/2}|^2 + |a_{-1/2}|^2 = 1. }[/math]
For a generic particle with spin s, we would need 2s + 1 such parameters. Since these numbers depend on the choice of the axis, they transform into each other non-trivially when this axis is rotated. It is clear that the transformation law must be linear, so we can represent it by associating a matrix with each rotation, and the product of two transformation matrices corresponding to rotations A and B must be equal (up to phase) to the matrix representing rotation AB. Further, rotations preserve the quantum-mechanical inner product, and so should our transformation matrices: [math]\displaystyle{ \sum_{m=-j}^j a_m^* b_m = \sum_{m=-j}^j \left(\sum_{n=-j}^j U_{nm} a_n\right)^* \left(\sum_{k=-j}^j U_{km} b_k\right), }[/math] [math]\displaystyle{ \sum_{n=-j}^j \sum_{k=-j}^j U_{np}^* U_{kq} = \delta_{pq}. }[/math]
Mathematically speaking, these matrices furnish a unitary projective representation of the rotation group SO(3). Each such representation corresponds to a representation of the covering group of SO(3), which is SU(2).[21] There is one n-dimensional irreducible representation of SU(2) for each dimension, though this representation is n-dimensional real for odd n and n-dimensional complex for even n (hence of real dimension 2n). For a rotation by angle θ in the plane with normal vector [math]\displaystyle{ \hat{\boldsymbol{\theta}} }[/math], [math]\displaystyle{ U = e^{-\frac{i}{\hbar} \boldsymbol{\theta} \cdot \mathbf{S}}, }[/math] where [math]\displaystyle{ \boldsymbol{\theta} = \theta \hat{\boldsymbol{\theta}} }[/math], and S is the vector of spin operators.
Working in the coordinate system where [math]\displaystyle{ \hat{\theta} = \hat{z} }[/math], we would like to show that Sx and Sy are rotated into each other by the angle θ. Starting with Sx. Using units where ħ = 1: [math]\displaystyle{ \begin{align} S_x \rightarrow U^\dagger S_x U &= e^{i \theta S_z} S_x e^{-i \theta S_z} \\ &= S_x + (i \theta) \left[S_z, S_x\right] + \left(\frac{1}{2!}\right) (i \theta)^2 \left[S_z, \left[S_z, S_x\right]\right] + \left(\frac{1}{3!}\right) (i \theta)^3 \left[S_z, \left[S_z, \left[S_z, S_x\right]\right]\right] + \cdots \end{align} }[/math]
Using the spin operator commutation relations, we see that the commutators evaluate to i Sy for the odd terms in the series, and to Sx for all of the even terms. Thus: [math]\displaystyle{ \begin{align} U^\dagger S_x U &= S_x \left[ 1 - \frac{\theta^2}{2!} + \cdots \right] - S_y \left[ \theta - \frac{\theta^3}{3!} \cdots \right] \\ &= S_x \cos\theta - S_y \sin\theta, \end{align} }[/math] as expected. Note that since we only relied on the spin operator commutation relations, this proof holds for any dimension (i.e., for any principal spin quantum number s)[22]:164
A generic rotation in 3-dimensional space can be built by compounding operators of this type using Euler angles: [math]\displaystyle{ \mathcal{R}(\alpha, \beta, \gamma) = e^{-i\alpha S_x} e^{-i\beta S_y} e^{-i\gamma S_z}. }[/math]
An irreducible representation of this group of operators is furnished by the Wigner D-matrix: [math]\displaystyle{ D^s_{m'm}(\alpha, \beta, \gamma) \equiv \langle sm' | \mathcal{R}(\alpha, \beta, \gamma) | sm \rangle = e^{-im'\alpha} d^s_{m'm}(\beta)e^{-i m\gamma}, }[/math] where [math]\displaystyle{ d^s_{m'm}(\beta) = \langle sm' | e^{-i\beta s_y} | sm \rangle }[/math] is Wigner's small d-matrix. Note that for γ = 2π and α = β = 0; i.e., a full rotation about the z axis, the Wigner D-matrix elements become [math]\displaystyle{ D^s_{m'm}(0, 0, 2\pi) = d^s_{m'm}(0) e^{-i m 2 \pi} = \delta_{m'm} (-1)^{2m}. }[/math]
Recalling that a generic spin state can be written as a superposition of states with definite m, we see that if s is an integer, the values of m are all integers, and this matrix corresponds to the identity operator. However, if s is a half-integer, the values of m are also all half-integers, giving (−1)2m = −1 for all m, and hence upon rotation by 2π the state picks up a minus sign. This fact is a crucial element of the proof of the spin–statistics theorem.
Lorentz transformations
We could try the same approach to determine the behavior of spin under general Lorentz transformations, but we would immediately discover a major obstacle. Unlike SO(3), the group of Lorentz transformations SO(3,1) is non-compact and therefore does not have any faithful, unitary, finite-dimensional representations.
In case of spin-1/2 particles, it is possible to find a construction that includes both a finite-dimensional representation and a scalar product that is preserved by this representation. We associate a 4-component Dirac spinor ψ with each particle. These spinors transform under Lorentz transformations according to the law [math]\displaystyle{ \psi' = \exp{\left(\tfrac{1}{8} \omega_{\mu\nu} [\gamma_{\mu}, \gamma_{\nu}]\right)} \psi, }[/math] where γν are gamma matrices, and ωμν is an antisymmetric 4 × 4 matrix parametrizing the transformation. It can be shown that the scalar product [math]\displaystyle{ \langle\psi|\phi\rangle = \bar{\psi}\phi = \psi^\dagger \gamma_0 \phi }[/math] is preserved. It is not, however, positive-definite, so the representation is not unitary.
Measurement of spin along the x, y, or z axes
Each of the (Hermitian) Pauli matrices of spin-1/2 particles has two eigenvalues, +1 and −1. The corresponding normalized eigenvectors are [math]\displaystyle{ \begin{array}{lclc} \psi_{x+} = \left|\frac{1}{2}, \frac{+1}{2}\right\rangle_x = \displaystyle\frac{1}{\sqrt{2}} \!\!\!\!\! & \begin{pmatrix}{1}\\{1}\end{pmatrix}, & \psi_{x-} = \left|\frac{1}{2}, \frac{-1}{2}\right\rangle_x = \displaystyle\frac{1}{\sqrt{2}} \!\!\!\!\! & \begin{pmatrix}{1}\\{-1}\end{pmatrix}, \\ \psi_{y+} = \left|\frac{1}{2}, \frac{+1}{2}\right\rangle_y = \displaystyle\frac{1}{\sqrt{2}} \!\!\!\!\! & \begin{pmatrix}{1}\\{i}\end{pmatrix}, & \psi_{y-} = \left|\frac{1}{2}, \frac{-1}{2}\right\rangle_y = \displaystyle\frac{1}{\sqrt{2}} \!\!\!\!\! & \begin{pmatrix}{1}\\{-i}\end{pmatrix}, \\ \psi_{z+} = \left|\frac{1}{2}, \frac{+1}{2}\right\rangle_z = & \begin{pmatrix} 1 \\ 0 \end{pmatrix}, & \psi_{z-} = \left|\frac{1}{2}, \frac{-1}{2}\right\rangle_z = & \begin{pmatrix} 0 \\ 1 \end{pmatrix}. \end{array} }[/math]
(Because any eigenvector multiplied by a constant is still an eigenvector, there is ambiguity about the overall sign. In this article, the convention is chosen to make the first element imaginary and negative if there is a sign ambiguity. The present convention is used by software such as SymPy; while many physics textbooks, such as Sakurai and Griffiths, prefer to make it real and positive.)
By the postulates of quantum mechanics, an experiment designed to measure the electron spin on the x, y, or z axis can only yield an eigenvalue of the corresponding spin operator (Sx, Sy or Sz) on that axis, i.e. ħ/2 or –ħ/2. The quantum state of a particle (with respect to spin), can be represented by a two-component spinor: [math]\displaystyle{ \psi = \begin{pmatrix} a + bi \\ c + di \end{pmatrix}. }[/math]
When the spin of this particle is measured with respect to a given axis (in this example, the x axis), the probability that its spin will be measured as ħ/2 is just [math]\displaystyle{ \big|\langle \psi_{x+}|\psi\rangle\big|^2 }[/math]. Correspondingly, the probability that its spin will be measured as –ħ/2 is just [math]\displaystyle{ \big|\langle\psi_{x-}|\psi\rangle\big|^2 }[/math]. Following the measurement, the spin state of the particle collapses into the corresponding eigenstate. As a result, if the particle's spin along a given axis has been measured to have a given eigenvalue, all measurements will yield the same eigenvalue (since [math]\displaystyle{ \big|\langle\psi_{x+} | \psi_{x+}\rangle\big|^2 = 1 }[/math], etc.), provided that no measurements of the spin are made along other axes.
Measurement of spin along an arbitrary axis
The operator to measure spin along an arbitrary axis direction is easily obtained from the Pauli spin matrices. Let u = (ux, uy, uz) be an arbitrary unit vector. Then the operator for spin in this direction is simply [math]\displaystyle{ S_u = \frac{\hbar}{2} (u_x \sigma_x + u_y \sigma_y + u_z \sigma_z). }[/math]
The operator Su has eigenvalues of ±ħ/2, just like the usual spin matrices. This method of finding the operator for spin in an arbitrary direction generalizes to higher spin states, one takes the dot product of the direction with a vector of the three operators for the three x-, y-, z-axis directions.
A normalized spinor for spin-1/2 in the (ux, uy, uz) direction (which works for all spin states except spin down, where it will give 0/0) is [math]\displaystyle{ \frac{1}{\sqrt{2 + 2u_z}} \begin{pmatrix} 1 + u_z \\ u_x + iu_y \end{pmatrix}. }[/math]
The above spinor is obtained in the usual way by diagonalizing the σu matrix and finding the eigenstates corresponding to the eigenvalues. In quantum mechanics, vectors are termed "normalized" when multiplied by a normalizing factor, which results in the vector having a length of unity.
Compatibility of spin measurements
Since the Pauli matrices do not commute, measurements of spin along the different axes are incompatible. This means that if, for example, we know the spin along the x axis, and we then measure the spin along the y axis, we have invalidated our previous knowledge of the x axis spin. This can be seen from the property of the eigenvectors (i.e. eigenstates) of the Pauli matrices that [math]\displaystyle{ \big| \langle \psi_{x\pm} | \psi_{y\pm} \rangle \big|^2 = \big| \langle \psi_{x\pm} | \psi_{z\pm} \rangle \big|^2 = \big| \langle \psi_{y\pm} | \psi_{z\pm} \rangle \big|^2 = \tfrac{1}{2}. }[/math]
So when physicists measure the spin of a particle along the x axis as, for example, ħ/2, the particle's spin state collapses into the eigenstate [math]\displaystyle{ |\psi_{x+}\rangle }[/math]. When we then subsequently measure the particle's spin along the y axis, the spin state will now collapse into either [math]\displaystyle{ |\psi_{y+}\rangle }[/math] or [math]\displaystyle{ |\psi_{y-}\rangle }[/math], each with probability 1/2. Let us say, in our example, that we measure −ħ/2. When we now return to measure the particle's spin along the x axis again, the probabilities that we will measure ħ/2 or −ħ/2 are each 1/2 (i.e. they are [math]\displaystyle{ \big| \langle \psi_{x+} | \psi_{y-} \rangle \big|^2 }[/math] and [math]\displaystyle{ \big| \langle \psi_{x-} | \psi_{y-} \rangle \big|^2 }[/math] respectively). This implies that the original measurement of the spin along the x axis is no longer valid, since the spin along the x axis will now be measured to have either eigenvalue with equal probability.
Higher spins
The spin-1/2 operator S = ħ/2σ forms the fundamental representation of SU(2). By taking Kronecker products of this representation with itself repeatedly, one may construct all higher irreducible representations. That is, the resulting spin operators for higher-spin systems in three spatial dimensions can be calculated for arbitrarily large s using this spin operator and ladder operators. For example, taking the Kronecker product of two spin-1/2 yields a four-dimensional representation, which is separable into a 3-dimensional spin-1 (triplet states) and a 1-dimensional spin-0 representation (singlet state).
The resulting irreducible representations yield the following spin matrices and eigenvalues in the z-basis:
- For spin 1 they are [math]\displaystyle{ \begin{align} S_x &= \frac{\hbar}{\sqrt{2}} \begin{pmatrix} 0 & 1 & 0 \\ 1 & 0 & 1 \\ 0 & 1 & 0 \end{pmatrix}, & \left|1, +1\right\rangle_x &= \frac{1}{2} \begin{pmatrix} 1 \\{\sqrt{2}}\\ 1 \end{pmatrix}, & \left|1, 0\right\rangle_x &= \frac{1}{\sqrt{2}} \begin{pmatrix} -1 \\ 0 \\ 1 \end{pmatrix}, & \left|1, -1\right\rangle_x &= \frac{1}{2} \begin{pmatrix} 1 \\{-\sqrt{2}}\\ 1 \end{pmatrix} \\ S_y &= \frac{\hbar}{\sqrt{2}} \begin{pmatrix} 0 & -i & 0 \\ i & 0 & -i \\ 0 & i & 0 \end{pmatrix}, & \left|1, +1\right\rangle_y &= \frac{1}{2} \begin{pmatrix} -1 \\ -i\sqrt{2} \\ 1 \end{pmatrix}, & \left|1, 0\right\rangle_y &= \frac{1}{\sqrt{2}} \begin{pmatrix} 1 \\ 0 \\ 1 \end{pmatrix}, & \left|1, -1\right\rangle_y &= \frac{1}{2} \begin{pmatrix} -1 \\ i\sqrt{2} \\ 1 \end{pmatrix} \\ S_z &= \hbar \begin{pmatrix} 1 & 0 & 0 \\ 0 & 0 & 0 \\ 0 & 0 & -1 \end{pmatrix}, & \left|1, +1\right\rangle_z &= \begin{pmatrix} 1 \\ 0 \\ 0 \end{pmatrix}, & \left|1, 0\right\rangle_z &= \begin{pmatrix} 0 \\ 1 \\ 0 \end{pmatrix}, & \left|1, -1\right\rangle_z &= \begin{pmatrix} 0 \\ 0 \\ 1 \end{pmatrix} \\ \end{align} }[/math]
- For spin 3/2 they are [math]\displaystyle{ \begin{array}{lclc} S_x = \frac\hbar2 \begin{pmatrix} 0 &\sqrt{3} &0 &0\\ \sqrt{3} &0 &2 &0\\ 0 &2 &0 &\sqrt{3}\\ 0 &0 &\sqrt{3} &0 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{+3}{2}\right\rangle_x =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix} 1 \\{\sqrt{3}}\\{\sqrt{3}}\\ 1 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{+1}{2}\right\rangle_x =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix}{-\sqrt{3}}\\ -1 \\ 1 \\{\sqrt{3}}\end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{-1}{2}\right\rangle_x =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix}{\sqrt{3}}\\ -1 \\ -1 \\{\sqrt{3}}\end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{-3}{2}\right\rangle_x =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix} -1 \\{\sqrt{3}}\\{-\sqrt{3}}\\ 1 \end{pmatrix} \\ S_y = \frac\hbar2 \begin{pmatrix} 0 &-i\sqrt{3} &0 &0\\ i\sqrt{3} &0 &-2i &0\\ 0 &2i &0 &-i\sqrt{3}\\ 0 &0 &i\sqrt{3} &0 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{+3}{2}\right\rangle_y =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix}{i}\\{-\sqrt{3}}\\{-i\sqrt{3}}\\ 1 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{+1}{2}\right\rangle_y =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix}{-i\sqrt{3}}\\ 1 \\{-i}\\{\sqrt{3}}\end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{-1}{2}\right\rangle_y =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix}{i\sqrt{3}}\\ 1 \\{i}\\{\sqrt{3}}\end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{-3}{2}\right\rangle_y =\!\!\! & \frac{1}{2\sqrt{2}} \begin{pmatrix}{-i}\\{-\sqrt{3}}\\{i\sqrt{3}}\\ 1 \end{pmatrix} \\ S_z = \frac\hbar2 \begin{pmatrix} 3 &0 &0 &0\\ 0 &1 &0 &0\\ 0 &0 &-1 &0\\ 0 &0 &0 &-3 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{+3}{2}\right\rangle_z =\!\!\! & \begin{pmatrix} 1 \\ 0 \\ 0 \\ 0 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{+1}{2}\right\rangle_z =\!\!\! & \begin{pmatrix} 0 \\ 1 \\ 0 \\ 0 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{-1}{2}\right\rangle_z =\!\!\! & \begin{pmatrix} 0 \\ 0 \\ 1 \\ 0 \end{pmatrix}, \!\!\! & \left|\frac{3}{2}, \frac{-3}{2}\right\rangle_z =\!\!\! & \begin{pmatrix} 0 \\ 0 \\ 0 \\ 1 \end{pmatrix} \\ \end{array} }[/math]
- For spin 5/2 they are [math]\displaystyle{ \begin{align} \boldsymbol{S}_x &= \frac{\hbar}{2} \begin{pmatrix} 0 &\sqrt{5} &0 &0 &0 &0 \\ \sqrt{5} &0 &2\sqrt{2} &0 &0 &0 \\ 0 &2\sqrt{2} &0 &3 &0 &0 \\ 0 &0 &3 &0 &2\sqrt{2} &0 \\ 0 &0 &0 &2\sqrt{2} &0 &\sqrt{5} \\ 0 &0 &0 &0 &\sqrt{5} &0 \end{pmatrix}, \\ \boldsymbol{S}_y &= \frac{\hbar}{2} \begin{pmatrix} 0 &-i\sqrt{5} &0 &0 &0 &0 \\ i\sqrt{5} &0 &-2i\sqrt{2} &0 &0 &0 \\ 0 &2i\sqrt{2} &0 &-3i &0 &0 \\ 0 &0 &3i &0 &-2i\sqrt{2} &0 \\ 0 &0 &0 &2i\sqrt{2} &0 &-i\sqrt{5} \\ 0 &0 &0 &0 &i\sqrt{5} &0 \end{pmatrix}, \\ \boldsymbol{S}_z &= \frac{\hbar}{2} \begin{pmatrix} 5 &0 &0 &0 &0 &0 \\ 0 &3 &0 &0 &0 &0 \\ 0 &0 &1 &0 &0 &0 \\ 0 &0 &0 &-1 &0 &0 \\ 0 &0 &0 &0 &-3 &0 \\ 0 &0 &0 &0 &0 &-5 \end{pmatrix}. \end{align} }[/math]
- The generalization of these matrices for arbitrary spin s is [math]\displaystyle{ \begin{align} \left(S_x\right)_{ab} & = \frac{\hbar}{2} \left(\delta_{a,b+1} + \delta_{a+1,b}\right) \sqrt{(s + 1)(a + b - 1) - ab}, \\ \left(S_y\right)_{ab} & = \frac{i\hbar}{2} \left(\delta_{a,b+1} - \delta_{a+1,b}\right) \sqrt{(s + 1)(a + b - 1) - ab}, \\ \left(S_z\right)_{ab} & = \hbar (s + 1 - a) \delta_{a,b} = \hbar (s + 1 - b) \delta_{a,b}, \end{align} }[/math] where indices [math]\displaystyle{ a, b }[/math] are integer numbers such that [math]\displaystyle{ 1 \le a \le 2s + 1, \quad 1 \le b \le 2s + 1. }[/math]
Also useful in the quantum mechanics of multiparticle systems, the general Pauli group Gn is defined to consist of all n-fold tensor products of Pauli matrices.
The analog formula of Euler's formula in terms of the Pauli matrices [math]\displaystyle{ \hat{R}(\theta, \hat{\mathbf{n}}) = e^{i \frac{\theta}{2} \hat{\mathbf{n}} \cdot \boldsymbol{\sigma}} = I \cos \frac{\theta}{2} + i \left(\hat{\mathbf{n}} \cdot \boldsymbol{\sigma}\right) \sin \frac{\theta}{2} }[/math] for higher spins is tractable, but less simple.[23]
Parity
In tables of the spin quantum number s for nuclei or particles, the spin is often followed by a "+" or "−". This refers to the parity with "+" for even parity (wave function unchanged by spatial inversion) and "−" for odd parity (wave function negated by spatial inversion). For example, see the isotopes of bismuth, in which the list of isotopes includes the column nuclear spin and parity. For Bi-209, the longest-lived isotope, the entry 9/2– means that the nuclear spin is 9/2 and the parity is odd.
Applications
Spin has important theoretical implications and practical applications. Well-established direct applications of spin include:
- Nuclear magnetic resonance (NMR) spectroscopy in chemistry;
- Electron spin resonance (ESR or EPR) spectroscopy in chemistry and physics;
- Magnetic resonance imaging (MRI) in medicine, a type of applied NMR, which relies on proton spin density;
- Giant magnetoresistive (GMR) drive-head technology in modern hard disks.
Electron spin plays an important role in magnetism, with applications for instance in computer memories. The manipulation of nuclear spin by radio-frequency waves (nuclear magnetic resonance) is important in chemical spectroscopy and medical imaging.
Spin–orbit coupling leads to the fine structure of atomic spectra, which is used in atomic clocks and in the modern definition of the second. Precise measurements of the g-factor of the electron have played an important role in the development and verification of quantum electrodynamics. Photon spin is associated with the polarization of light (photon polarization).
An emerging application of spin is as a binary information carrier in spin transistors. The original concept, proposed in 1990, is known as Datta–Das spin transistor.[24] Electronics based on spin transistors are referred to as spintronics. The manipulation of spin in dilute magnetic semiconductor materials, such as metal-doped ZnO or TiO2 imparts a further degree of freedom and has the potential to facilitate the fabrication of more efficient electronics.[25]
There are many indirect applications and manifestations of spin and the associated Pauli exclusion principle, starting with the periodic table of chemistry.
History
Spin was first discovered in the context of the emission spectrum of alkali metals. In 1924, Wolfgang Pauli introduced what he called a "two-valuedness not describable classically"[26] associated with the electron in the outermost shell. This allowed him to formulate the Pauli exclusion principle, stating that no two electrons can have the same quantum state in the same quantum system.
The physical interpretation of Pauli's "degree of freedom" was initially unknown. Ralph Kronig, one of Alfred Landé's assistants, suggested in early 1925 that it was produced by the self-rotation of the electron. When Pauli heard about the idea, he criticized it severely, noting that the electron's hypothetical surface would have to be moving faster than the speed of light in order for it to rotate quickly enough to produce the necessary angular momentum. This would violate the theory of relativity. Largely due to Pauli's criticism, Kronig decided not to publish his idea.[27]
In the autumn of 1925, the same thought came to Dutch physicists George Uhlenbeck and Samuel Goudsmit at Leiden University. Under the advice of Paul Ehrenfest, they published their results.[28] It met a favorable response, especially after Llewellyn Thomas managed to resolve a factor-of-two discrepancy between experimental results and Uhlenbeck and Goudsmit's calculations (and Kronig's unpublished results). This discrepancy was due to the orientation of the electron's tangent frame, in addition to its position.
Mathematically speaking, a fiber bundle description is needed. The tangent bundle effect is additive and relativistic; that is, it vanishes if c goes to infinity. It is one half of the value obtained without regard for the tangent-space orientation, but with opposite sign. Thus the combined effect differs from the latter by a factor two (Thomas precession, known to Ludwik Silberstein in 1914).
Despite his initial objections, Pauli formalized the theory of spin in 1927, using the modern theory of quantum mechanics invented by Erwin Schrödinger and Werner Heisenberg. He pioneered the use of Pauli matrices as a representation of the spin operators and introduced a two-component spinor wave-function. Uhlenbeck and Goudsmit treated spin as arising from classical rotation, while Pauli emphasized, that spin is a non-classical and intrinsic property.[29]
Pauli's theory of spin was non-relativistic. However, in 1928, Paul Dirac published the Dirac equation, which described the relativistic electron. In the Dirac equation, a four-component spinor (known as a "Dirac spinor") was used for the electron wave-function. Relativistic spin explained gyromagnetic anomaly, which was (in retrospect) first observed by Samuel Jackson Barnett in 1914 (see Einstein–de Haas effect). In 1940, Pauli proved the spin–statistics theorem, which states that fermions have half-integer spin, and bosons have integer spin.
In retrospect, the first direct experimental evidence of the electron spin was the Stern–Gerlach experiment of 1922. However, the correct explanation of this experiment was only given in 1927.[30]
See also
- Chirality
- Dynamic nuclear polarization
- Helicity (particle physics)
- Holstein–Primakoff transformation
- Kramers' theorem
- Pauli equation
- Pauli–Lubanski pseudovector
- Rarita–Schwinger equation
- Representation theory of SU(2)
- Spin angular momentum of light
- Spin engineering
- Spin-flip
- Spin isomers of hydrogen
- Spin–orbit interaction
- Spin tensor
- Spin wave
- Yrast
References
- ↑ Merzbacher, Eugen (1998). Quantum Mechanics (3rd ed.). John Wiley & Sons. pp. 372–373. ISBN 978-0-471-88702-7. https://archive.org/details/quantummechanics00merz_136.
- ↑ 2.0 2.1 2.2 2.3 Griffiths, David (2005). Introduction to Quantum Mechanics (2nd ed.). https://archive.org/details/introductiontoqu00grif_190.
- ↑ 3.0 3.1 3.2 3.3 Ohanian, Hans C. (1986-06-01). "What is spin?" (in en). American Journal of Physics 54 (6): 500–505. doi:10.1119/1.14580. ISSN 0002-9505. Bibcode: 1986AmJPh..54..500O. https://physics.mcmaster.ca/phys3mm3/notes/whatisspin.pdf.
- ↑ Eisberg, Robert; Resnick, Robert (1985). Quantum Physics of Atoms, Molecules, Solids, Nuclei, and Particles (2nd ed.). Wiley. pp. 272–273. ISBN 978-0-471-87373-0. https://archive.org/details/quantumphysicsat00eisb.
- ↑ "Fermilab Today". https://www.fnal.gov/pub/today/archive/archive_2013/today13-02-15_NutshellReadMore.html.
- ↑ Bliokh, Konstantin Y.; Punzmann, Horst; Xia, Hua; Nori, Franco; Shats, Michael (2022-01-21). "Field theory spin and momentum in water waves" (in en). Science Advances 8 (3): eabm1295. doi:10.1126/sciadv.abm1295. ISSN 2375-2548. PMID 35061526. Bibcode: 2022SciA....8.1295B.
- ↑ Whittaker, Sir Edmund (1989-01-01). A History of the Theories of Aether and Electricity. 2. Courier Dover Publications. p. 87. ISBN 0-486-26126-3.
- ↑ Peskin, M. E., & Schroeder, D. V. (1995). Quantum field theory, Ch. 3. The Advanced Book Program.
- ↑ Sebens, Charles T. (November 2019). "How electrons spin" (in en). Studies in History and Philosophy of Science Part B: Studies in History and Philosophy of Modern Physics 68: 40–50. doi:10.1016/j.shpsb.2019.04.007. https://linkinghub.elsevier.com/retrieve/pii/S135521981830087X.
- ↑ Information about Higgs Boson in CERN's official website.
- ↑ Pauli, Wolfgang (1940). "The Connection Between Spin and Statistics". Phys. Rev. 58 (8): 716–722. doi:10.1103/PhysRev.58.716. Bibcode: 1940PhRv...58..716P. http://hermes.ffn.ub.es/luisnavarro/nuevo_maletin/Pauli_spin_statistics_1940.pdf.
- ↑ Physics of Atoms and Molecules, B. H. Bransden, C. J. Joachain, Longman, 1983, ISBN:0-582-44401-2.
- ↑ "2018 CODATA Value: electron g factor". The NIST Reference on Constants, Units, and Uncertainty. NIST. 20 May 2019. http://physics.nist.gov/cgi-bin/cuu/Value?gem. Retrieved 2019-05-20.
- ↑ Feynman, R. P. (1985). "Electrons and their interactions". QED: The Strange Theory of Light and Matter. Princeton, New Jersey: Princeton University Press. p. 115. ISBN 978-0-691-08388-9. "After some years, it was discovered that this value [−1/2 g] was not exactly 1, but slightly more – something like 1.00116. This correction was worked out for the first time in 1948 by Schwinger as j × j divided by 2π [sic] [where j is the square root of the fine-structure constant], and was due to an alternative way the electron can go from place to place: Instead of going directly from one point to another, the electron goes along for a while and suddenly emits a photon; then (horrors!) it absorbs its own photon."
- ↑ Marciano, W. J.; Sanda, A. I. (1977). "Exotic decays of the muon and heavy leptons in gauge theories". Physics Letters B67 (3): 303–305. doi:10.1016/0370-2693(77)90377-X. Bibcode: 1977PhLB...67..303M.
- ↑ Lee, B. W.; Shrock, R. E. (1977). "Natural suppression of symmetry violation in gauge theories: Muon- and electron-lepton-number nonconservation". Physical Review D16 (5): 1444–1473. doi:10.1103/PhysRevD.16.1444. Bibcode: 1977PhRvD..16.1444L.
- ↑ K. Fujikawa; R. E. Shrock (1980). "Magnetic Moment of a Massive Neutrino and Neutrino-Spin Rotation". Physical Review Letters 45 (12): 963–966. doi:10.1103/PhysRevLett.45.963. Bibcode: 1980PhRvL..45..963F.
- ↑ Bell, N. F. et al. (2005). "How Magnetic is the Dirac neutrino?". Physical Review Letters 95 (15): 151802. doi:10.1103/PhysRevLett.95.151802. PMID 16241715. Bibcode: 2005PhRvL..95o1802B.
- ↑ Quanta: A handbook of concepts, P. W. Atkins, Oxford University Press, 1974, ISBN:0-19-855493-1.
- ↑ Messiah, Albert (2014). "Angular Momentum in Quantum Mechanics" (in en). Quantum Mechanics. Mineola, NY: Dover Publications. p. 540. ISBN 978-1-306-51279-4. OCLC 874097814.
- ↑ B. C. Hall (2013). Quantum Theory for Mathematicians. Springer. pp. 354–358.
- ↑ Sakurai, Jun John; Napolitano, Jim (2017). Modern quantum mechanics (2nd ed.). Cambridge: Cambridge university press. ISBN 978-1-108-42241-3. https://www.academia.edu/download/64391583/modern%20quantum%20mechanics.pdf.
- ↑ Curtright, T. L.; Fairlie, D. B.; Zachos, C. K. (2014). "A compact formula for rotations as spin matrix polynomials". SIGMA 10: 084. doi:10.3842/SIGMA.2014.084. Bibcode: 2014SIGMA..10..084C.
- ↑ Datta, S.; Das, B. (1990). "Electronic analog of the electrooptic modulator". Applied Physics Letters 56 (7): 665–667. doi:10.1063/1.102730. Bibcode: 1990ApPhL..56..665D.
- ↑ Assadi, M. H. N.; Hanaor, D. A. H. (2013). "Theoretical study on copper's energetics and magnetism in TiO2 polymorphs". Journal of Applied Physics 113 (23): 233913–233913–5. doi:10.1063/1.4811539. Bibcode: 2013JAP...113w3913A.
- ↑ Wolfgang Pauli (December 13, 1946). "Exclusion Principle and Quantum Mechanics". Nobel Lecture. Nobel Prize. https://www.nobelprize.org/prizes/physics/1945/pauli/lecture/.
- ↑ Pais, Abraham (1991). Niels Bohr's Times. Oxford: Clarendon Press. pp. 244. ISBN 978-0-19-852049-8. https://archive.org/details/nielsbohrstimesi00pais_0.
- ↑ Ehrenfest, P. (November 1925). "Ersetzung der Hypothese vom unmechanischen Zwang durch eine Forderung bezüglich des inneren Verhaltens jedes einzelnen Elektrons" (in de). Die Naturwissenschaften 13 (47): 953–954. doi:10.1007/bf01558878. ISSN 0028-1042.
- ↑ Ohanian, Hans C. (June 1986). "What is spin?" (in en). American Journal of Physics 54 (6): 500–505. doi:10.1119/1.14580. ISSN 0002-9505. Bibcode: 1986AmJPh..54..500O. http://aapt.scitation.org/doi/10.1119/1.14580.
- ↑ B. Friedrich; D. Herschbach (2003). "Stern and Gerlach: How a Bad Cigar Helped Reorient Atomic Physics". Physics Today 56 (12): 53. doi:10.1063/1.1650229. Bibcode: 2003PhT....56l..53F.
Further reading
- Cohen-Tannoudji, Claude; Diu, Bernard; Laloë, Franck (2006). Quantum Mechanics (2 volume set ed.). John Wiley & Sons. ISBN 978-0-471-56952-7.
- Condon, E. U.; Shortley, G. H. (1935). "Especially Chapter 3". The Theory of Atomic Spectra. Cambridge University Press. ISBN 978-0-521-09209-8.
- Hipple, J. A.; Sommer, H.; Thomas, H.A. (1949). "A precise method of determining the faraday by magnetic resonance". Physical Review 76 (12): 1877–1878. doi:10.1103/PhysRev.76.1877.2. Bibcode: 1949PhRv...76.1877H. https://www.academia.edu/6483539.
- Edmonds, A. R. (1957). Angular Momentum in Quantum Mechanics. Princeton University Press. ISBN 978-0-691-07912-7. https://archive.org/details/angularmomentumi0000edmo.
- Jackson, John David (1998). Classical Electrodynamics (3rd ed.). John Wiley & Sons. ISBN 978-0-471-30932-1.
- Serway, Raymond A.; Jewett, John W. (2004). Physics for Scientists and Engineers (6th ed.). Brooks/Cole. ISBN 978-0-534-40842-8. https://archive.org/details/physicssciengv2p00serw.
- Thompson, William J. (1994). Angular Momentum: An Illustrated Guide to Rotational Symmetries for Physical Systems. Wiley. ISBN 978-0-471-55264-2.
- Tipler, Paul (2004). Physics for Scientists and Engineers: Mechanics, Oscillations and Waves, Thermodynamics (5th ed.). W. H. Freeman. ISBN 978-0-7167-0809-4.
- Sin-Itiro Tomonaga, The Story of Spin, 1997
External links
- Goudsmit on the discovery of electron spin.
- Nature: "Milestones in 'spin' since 1896."
- ECE 495N Lecture 36: Spin Online lecture by S. Datta