Chemistry:Rubidium fluoride
| |
| Names | |
|---|---|
| Other names
Rubidium(I) Fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| RbF | |
| Molar mass | 104.4662 g/mol |
| Appearance | white crystalline solid |
| Density | 3.557 g/cm3 |
| Melting point | 795 °C (1,463 °F; 1,068 K) |
| Boiling point | 1,408 °C (2,566 °F; 1,681 K) |
| 130.6 g/100 mL (18 °C) | |
| −31.9·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Toxic |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Rubidium chloride Rubidium bromide Rubidium iodide Rubidium astatide |
Other cations
|
Lithium fluoride Sodium fluoride Potassium fluoride Caesium fluoride Francium fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
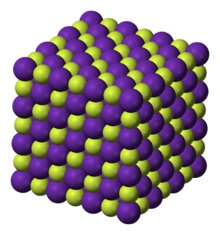
Rubidium fluoride (RbF) is the fluoride salt of rubidium. It is a cubic crystal with rock-salt structure.
Synthesis
There are several methods for synthesising rubidium fluoride. One involves reacting rubidium hydroxide with hydrofluoric acid:[1]
- RbOH + HF → RbF + H2O
Another method is to neutralize rubidium carbonate with hydrofluoric acid:[1]
- Rb2CO3 + 2HF → 2RbF + H2O + CO2
Another possible method is to react rubidium hydroxide with ammonium fluoride:
- RbOH + NH4F → RbF + H2O + NH3
The least used method due to expense of rubidium metal is to react it directly with fluorine gas, as rubidium reacts violently with halogens:[1]
- 2Rb + F2 → 2RbF
Properties
Rubidium fluoride is a white crystalline substance with a cubic crystal structure that looks very similar to common salt (NaCl). The crystals belong to the space group Fm3m (space group no. 225) with the lattice parameter a = 565 pm and four formula units per unit cell.[2] The refractive index of the crystals is nD = 1.398.[2] Rubidium fluoride colors a flame (Bunsen burner flame) purple or magenta red (spectral analysis).
Rubidium fluoride forms two different hydrates, a sesquihydrate with the stoichiometric composition 2RbF·3H2O and a third hydrate with the composition 3RbF·H2O.[3]
In addition to simple rubidium fluoride, an acidic rubidium fluoride with the molecular formula HRbF2 is also known,[4] which can be produced by reacting rubidium fluoride and hydrogen fluoride.[4] The compounds H2RbF3 and H3RbF4 were also synthesized.[5][4]
The solubility in acetone is 0.0036 g/kg at 18 °C and 0.0039 g/kg at 37 °C.[6]
The standard enthalpy of formation of rubidium fluoride is ΔfH0298 = −552.2 kJ mol−1,[7] the standard free enthalpy of formation ΔG0298 = −520.4 kJ mol−1,[7] and the standard molar entropy S0298 = 113.9 J K −1 ·mol−1.[7] The enthalpy of solution of rubidium fluoride was determined to be −24.28 kJ/mol.[8]
References
- ↑ 1.0 1.1 1.2 "WebElements". http://www.webelements.com/webelements/compounds/text/Rb/F1Rb1-13446747.html. Retrieved 23 February 2006.
- ↑ 2.0 2.1 Ans, Jean d'; Lax, Ellen (1998) (in de). Taschenbuch für Chemiker und Physiker. Springer. ISBN 978-3-540-60035-0. https://books.google.com/books?id=oWjEKDnsJgEC&pg=PA686.
- ↑ texte, Académie des sciences (France) Auteur du (1911-01-01). "Comptes rendus hebdomadaires des séances de l'Académie des sciences / publiés... par MM. les secrétaires perpétuels" (in EN). https://gallica.bnf.fr/ark:/12148/bpt6k3105c.
- ↑ 4.0 4.1 4.2 Eggeling, Hans; Meyer, Jullius (1905-08-19). "Über die Fluoride des Rubidiums" (in en). Zeitschrift für anorganische Chemie 46 (1): 174–176. doi:10.1002/zaac.19050460111. ISSN 0863-1778. https://onlinelibrary.wiley.com/doi/10.1002/zaac.19050460111.
- ↑ (in en) A Text-Book of Inorganic Chemistry. Forgotten Books. ISBN 978-1-4510-0469-4. https://books.google.com/books?id=C42fSjVzmkAC&pg=PA209.
- ↑ Aterton Seidell (1940). Solubilities Of Organic Compounds Vol - I. Carnegie-Mellon University Hunt Library, N.Sathyanarayanan. D.Van Nostrand Co.. http://archive.org/details/solubilitiesofor023311mbp.
- ↑ 7.0 7.1 7.2 Dickerson, Richard E. (1988) (in de). Prinzipien der Chemie. Walter de Gruyter. ISBN 978-3-11-009969-0. https://books.google.com/books?id=F4c6AfwbTEAC&pg=PA976.
- ↑ texte, Académie des sciences (France) Auteur du (1911-01-01). "Comptes rendus hebdomadaires des séances de l'Académie des sciences / publiés... par MM. les secrétaires perpétuels" (in EN). https://gallica.bnf.fr/ark:/12148/bpt6k3105c.
- "Rubidium compounds: rubidium fluoride". WebElements: the periodic table on the web. WebElements. http://www.webelements.com/compounds/rubidium/rubidium_fluoride.html.
 |


