Biology:Steroid 11β-hydroxylase
 Generic protein structure example |
| steroid 11β-monooxygenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.14.15.4 | ||||||||
| CAS number | 9029-66-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
Steroid 11β-hydroxylase, also known as steroid 11β-monooxygenase, is a steroid hydroxylase found in the zona glomerulosa and zona fasciculata of the adrenal cortex. Named officially the cytochrome P450 11B1, mitochondrial, it is a protein that in humans is encoded by the CYP11B1 gene.[1][2] The enzyme is involved in the biosynthesis of adrenal corticosteroids[3] by catalyzing the addition of hydroxyl groups during oxidation reactions.
Gene
The CYP11B1 gene encodes 11β-hydroxylase, a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases that catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. The product of this CYP11B1 gene is the 11β-hydroxylase protein. This protein localizes to the mitochondrial inner membrane and is involved in the conversion of various steroids in the adrenal cortex. Transcript variants encoding different isoforms have been noted for this gene.[2]
The CYP11B1 gene is reversibly inhibited by etomidate[4][5] and metyrapone.
Function
11β-hydroxylase is a steroidogenic enzyme, i.e. the enzyme involved in the metabolism of steroids. The enzyme is primarily localized in the zona glomerulosa and zona fasciculata of the adrenal cortex. The enzyme functions by introducing a hydroxyl group at carbon position 11β on the steroid nucleus, thereby facilitating the conversion of certain steroids.
Humans have two isozymes with 11β-hydroxylase activity: CYP11B1 and CYP11B2.
CYP11B1 (11β-hydroxylase) is expressed at high levels and is regulated by ACTH, while CYP11B2 (aldosterone synthase) is usually expressed at low levels and is regulated by angiotensin II. In addition to the 11β-hydroxylase activity, both isozymes have 18-hydroxylase activity.[6] The CYP11B1 isozyme has strong 11β-hydroxylase activity, but the activity of 18-hydroxylase is only one-tenth of CYP11B2.[7] The weak 18-hydroxylase activity of CYP11B1 explains why an adrenal with suppressed CYP11B2 expression continues to synthesize 18-hydroxycorticosterone.[8]
Here are some of the steroids, grouped by catalytic activity of the CYP11B1 isozyme:
- strong activity:[9][10]
- medium activity:[9][10]
- progesterone to 11β-hydroxyprogesterone,[10][13][14]
- 17α-hydroxyprogesterone to 21-deoxycortisol,[13]
- androstenedione to 11β-hydroxyandrostenedione;[15]
- testosterone to 11β-hydroxytestosterone,[16][17][10]
- weak activity:[9][10]
Cortisol and corticosterone metabolism
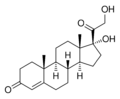
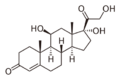
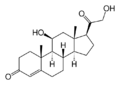
11β-hydroxylase has strong[9] catalytic activity during conversion of 11-deoxycortisol to cortisol and 11-deoxycorticosterone to corticosterone, by catalyzing the hydroxylation of carbon hydrogen bond at 11-beta position. Note the extra "–OH" added at the 11 position (near the center, on ring "C"):
Mechanism of action
As a mitochondrial P450 system, P450c11 is dependent on two electron transfer proteins, adrenodoxin reductase and adrenodoxin that transfer 2 electrons from NADPH to the P450 for each monooxygenase reaction catalyzed by the enzyme. In most respects this process of electron transfer appears similar to that of P450scc system that catalyzes cholesterol side chain cleavage.[21] Similar to P450scc the process of electrons transfer is leaky leading to superoxide production. The rate of electron leakage during metabolism depends on the functional groups of the steroid substrate.[22]
Regulation
The expression of the enzyme in adrenocortical cells is regulated by the trophic hormone corticotropin (ACTH).[23]
Clinical significance
A mutation in genes encoding 11β-hydroxylase is associated with congenital adrenal hyperplasia due to 11β-hydroxylase deficiency.
11β-hydroxylase is involved in the metabolism of 17α-hydroxyprogesterone to 21-deoxycortisol,[13] in cases of congenital adrenal hyperplasia due to 21-hydroxylase deficiency.[24][25]
See also
Additional images
References
- ↑ "Hereditary hypertension caused by chimaeric gene duplications and ectopic expression of aldosterone synthase". Nature Genetics 2 (1): 66–74. September 1992. doi:10.1038/ng0992-66. PMID 1303253.
- ↑ 2.0 2.1 Template:NCBI RefSeq
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Purification and functional characterization of human 11beta hydroxylase expressed in Escherichia coli". The FEBS Journal 275 (4): 799–810. February 2008. doi:10.1111/j.1742-4658.2008.06253.x. PMID 18215163.
- ↑ "Etomidate: a selective adrenocortical 11 beta-hydroxylase inhibitor". Klinische Wochenschrift 62 (21): 1011–3. November 1984. doi:10.1007/bf01711722. PMID 6096625.
- ↑ Pediatric Cardiac Anesthesia. Lippincott Williams & Wilkins. 7 December 2004. p. 68. ISBN 978-0-7817-5175-9. https://books.google.com/books?id=ymuHmUWuUsAC&pg=PA68. Retrieved 30 April 2012.
- ↑ "Disorders of steroid 11 beta-hydroxylase isozymes". Endocrine Reviews 15 (4): 421–38. August 1994. doi:10.1210/edrv-15-4-421. PMID 7988480.
- ↑ "Steroid 11β-Hydroxylase Isozymes (CYP11B1 and CYP11B2)". Cytochrome P450. Handbook of Experimental Pharmacology. 105. Springer. 1993. pp. 641–650. doi:10.1007/978-3-642-77763-9_41. ISBN 978-3-642-77765-3.
- ↑ 8.0 8.1 8.2 8.3 "Mineralocorticoid hypertension". Indian Journal of Endocrinology and Metabolism 15 Suppl 4 (8): S298–312. October 2011. doi:10.4103/2230-8210.86972. PMID 22145132.
- ↑ 9.0 9.1 9.2 9.3 9.4 "Structural insights into aldosterone synthase substrate specificity and targeted inhibition". Molecular Endocrinology 27 (2): 315–24. February 2013. doi:10.1210/me.2012-1287. PMID 23322723.
- ↑ 10.0 10.1 10.2 10.3 10.4 "The in vitro metabolism of 11β-hydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway". The Journal of Steroid Biochemistry and Molecular Biology 178: 203–212. April 2018. doi:10.1016/j.jsbmb.2017.12.014. PMID 29277707.
- ↑ "11beta-hydroxylase deficiency" (in pt). Arquivos Brasileiros de Endocrinologia e Metabologia 48 (5): 713–23. October 2004. doi:10.1590/s0004-27302004000500018. PMID 15761543.
- ↑ "Clinical perspectives in congenital adrenal hyperplasia due to 11β-hydroxylase deficiency". Endocrine 55 (1): 19–36. January 2017. doi:10.1007/s12020-016-1189-x. PMID 27928728.
- ↑ 13.0 13.1 13.2 "Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency". The Journal of Clinical Endocrinology and Metabolism 100 (6): 2283–90. June 2015. doi:10.1210/jc.2015-1023. PMID 25850025.
- ↑ "Alternative androgen pathways". WikiJournal of Medicine 10: X. 2023. doi:10.15347/WJM/2023.003.
- ↑ "11β-hydroxyandrostenedione returns to the steroid arena: biosynthesis, metabolism and function". Molecules (MDPI AG) 18 (11): 13228–44. October 2013. doi:10.3390/molecules181113228. PMID 24165582.
- ↑ "Canonical and Noncanonical Androgen Metabolism and Activity". Prostate Cancer. Advances in Experimental Medicine and Biology. 1210. Springer. 2019. pp. 239–277. doi:10.1007/978-3-030-32656-2_11. ISBN 978-3-030-32655-5. "CYP11B1 and 2 have also been shown to 11β-hydroxylate T, yielding 11β-hydroxytestosterone (11OHT), though the levels produced by the adrenal are low due to the limited availability of adrenal derived T"
- ↑ "11-Keto-testosterone and other androgens of adrenal origin". Physiological Research 69 (Suppl 2): S187–S192. September 2020. doi:10.33549/physiolres.934516. PMID 33094617.
- ↑ 18.0 18.1 "Studies on the origin of circulating 18-hydroxycortisol and 18-oxocortisol in normal human subjects". The Journal of Clinical Endocrinology and Metabolism 89 (9): 4628–33. September 2004. doi:10.1210/jc.2004-0379. PMID 15356073.
- ↑ "Mutation analysis of CYP11B1 and CYP11B2 in patients with increased 18-hydroxycortisol production". Molecular and Cellular Endocrinology 214 (1–2): 167–74. February 2004. doi:10.1016/j.mce.2003.10.056. PMID 15062555.
- ↑ "DIAGNOSIS OF ENDOCRINE DISEASE: 18-Oxocortisol and 18-hydroxycortisol: is there clinical utility of these steroids?". European Journal of Endocrinology 178 (1): R1–R9. January 2018. doi:10.1530/EJE-17-0563. PMID 28904009.
- ↑ "Mechanisms of ionic activation of adrenal mitochondrial cytochromes P-450scc and P-45011 beta". J. Biol. Chem. 256 (9): 4329–35. May 1981. doi:10.1016/S0021-9258(19)69437-8. PMID 6783659. http://www.jbc.org/content/256/9/4329.full.pdf.
- ↑ "Electron leakage from the adrenal cortex mitochondrial P450scc and P450c11 systems: NADPH and steroid dependence". Arch. Biochem. Biophys. 317 (2): 412–6. March 1995. doi:10.1006/abbi.1995.1182. PMID 7893157. https://zenodo.org/record/890751.
- ↑ "Mechanism of corticotropin and cAMP induction of mitochondrial cytochrome P450 system enzymes in adrenal cortex cells". J. Biol. Chem. 265 (33): 20602–8. November 1990. doi:10.1016/S0021-9258(17)30545-8. PMID 2173715. http://www.jbc.org/content/265/33/20602.full.pdf.
- ↑ "Congenital Adrenal Hyperplasia: Time to Replace 17OHP with 21-Deoxycortisol". Hormone Research in Paediatrics 91 (6): 416–420. 2019. doi:10.1159/000501396. PMID 31450227.
- ↑ "Best Practice for Identification of Classical 21-Hydroxylase Deficiency Should Include 21 Deoxycortisol Analysis with Appropriate Isomeric Steroid Separation". Int J Neonatal Screen 9 (4): 58. October 2023. doi:10.3390/ijns9040058. PMID 37873849.
Further reading
- "Twin genes and endocrine disease: CYP21 and CYP11B genes". Acta Endocrinol. 129 (2): 97–108. August 1993. doi:10.1530/acta.0.1290097. PMID 8372604.
- "Familial varieties of primary aldosteronism". Clin. Exp. Pharmacol. Physiol. 28 (12): 1087–90. December 2001. doi:10.1046/j.1440-1681.2001.03574.x. PMID 11903322.
- "Frame shift by insertion of 2 basepairs in codon 394 of CYP11B1 causes congenital adrenal hyperplasia due to steroid 11 beta-hydroxylase deficiency". J. Clin. Endocrinol. Metab. 75 (5): 1278–81. November 1992. doi:10.1210/jcem.75.5.1430088. PMID 1430088.
- "Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2". Proc. Natl. Acad. Sci. U.S.A. 89 (17): 8327–31. September 1992. doi:10.1073/pnas.89.17.8327. PMID 1518866. Bibcode: 1992PNAS...89.8327P.
- "Role of steroid 11 beta-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans". Proc. Natl. Acad. Sci. U.S.A. 89 (4): 1458–62. February 1992. doi:10.1073/pnas.89.4.1458. PMID 1741400. Bibcode: 1992PNAS...89.1458K.
- "A mutation in CYP11B1 (Arg-448----His) associated with steroid 11 beta-hydroxylase deficiency in Jews of Moroccan origin". J. Clin. Invest. 87 (5): 1664–7. May 1991. doi:10.1172/JCI115182. PMID 2022736.
- "Cloning of cDNA and genomic DNA for human cytochrome P-45011 beta". FEBS Lett. 269 (2): 345–9. September 1990. doi:10.1016/0014-5793(90)81190-Y. PMID 2401360.
- "Characterization of two genes encoding human steroid 11 beta-hydroxylase (P-450(11) beta)". J. Biol. Chem. 264 (35): 20961–7. December 1989. doi:10.1016/S0021-9258(19)30030-4. PMID 2592361.
- "Cloning of cDNA encoding steroid 11 beta-hydroxylase (P450c11)". Proc. Natl. Acad. Sci. U.S.A. 84 (20): 7193–7. October 1987. doi:10.1073/pnas.84.20.7193. PMID 3499608. Bibcode: 1987PNAS...84.7193C.
- "A nonsense mutation (TGG [Trp116]-->TAG [Stop]) in CYP11B1 causes steroid 11 beta-hydroxylase deficiency". J. Clin. Endocrinol. Metab. 77 (6): 1677–82. December 1993. doi:10.1210/jcem.77.6.7903314. PMID 7903314.
- "CYP11B1 mutations causing non-classic adrenal hyperplasia due to 11 beta-hydroxylase deficiency". Hum. Mol. Genet. 6 (11): 1829–34. October 1997. doi:10.1093/hmg/6.11.1829. PMID 9302260.
- "Characterization of single-nucleotide polymorphisms in coding regions of human genes". Nat. Genet. 22 (3): 231–8. July 1999. doi:10.1038/10290. PMID 10391209.
- "Patterns of single-nucleotide polymorphisms in candidate genes for blood-pressure homeostasis". Nat. Genet. 22 (3): 239–47. July 1999. doi:10.1038/10297. PMID 10391210.
- "Interaction of CYP11B1 (cytochrome P-45011 beta) with CYP11A1 (cytochrome P-450scc) in COS-1 cells". Eur. J. Biochem. 262 (3): 720–6. June 1999. doi:10.1046/j.1432-1327.1999.00414.x. PMID 10411633.
- "The C494F variant in the CYP11B1 gene is a sequence polymorphism in the Spanish population". J. Clin. Endocrinol. Metab. 84 (12): 4749. December 1999. doi:10.1210/jcem.84.12.6272-6. PMID 10599751.
- "Bilateral laparoscopic adrenalectomy for congenital adrenal hyperplasia with severe hypertension, resulting from two novel mutations in splice donor sites of CYP11B1". J. Clin. Endocrinol. Metab. 85 (11): 4060–8. November 2000. doi:10.1210/jcem.85.11.6897. PMID 11095433.
- "Effects of 18-hydroxylated steroids on corticosteroid production by human aldosterone synthase and 11beta-hydroxylase". J. Clin. Endocrinol. Metab. 86 (9): 4326–9. September 2001. doi:10.1210/jcem.86.9.7797. PMID 11549669.
- "Unequal crossing-over between aldosterone synthase and 11beta-hydroxylase genes causes congenital adrenal hyperplasia". J. Clin. Endocrinol. Metab. 86 (9): 4445–52. September 2001. doi:10.1210/jcem.86.9.7820. PMID 11549691.
External links
- Steroid+11-beta-hydroxylase at the US National Library of Medicine Medical Subject Headings (MeSH)
 |