Chemistry:Vanadium(II) chloride
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Vanadium(II) chloride
| |||
| Other names
Vanadous chloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| VCl2 | |||
| Molar mass | 121.847 g/mol | ||
| Appearance | pale green solid | ||
| Density | 3.230 g/cm3 | ||
| Melting point | 1,027 °C (1,881 °F; 1,300 K) | ||
| Boiling point | 1,506 °C (2,743 °F; 1,779 K) | ||
| soluble | |||
| +2410.0·10−6 cm3/mol | |||
| Structure | |||
| CdI2 | |||
| octahedral | |||
| Hazards | |||
| Main hazards | Reacts with oxygen rapidly | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H302, H314 | |||
| P260, P264, P270, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P330, P363, P405 | |||
| Related compounds | |||
Other anions
|
vanadium(II) fluoride, vanadium(II) bromide, vanadium(II) iodide | ||
Other cations
|
titanium(II) chloride, chromium(II) chloride | ||
Related compounds
|
vanadium(III) chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Vanadium(II) chloride is the inorganic compound with the formula VCl2, and is the most reduced vanadium chloride. Vanadium(II) chloride is an apple-green solid that dissolves in water to give purple solutions.[2]
Solid VCl2 is prepared by thermal decomposition of VCl3, which leaves a residue of VCl2:[2]
- 2 VCl3 → VCl2 + VCl4
VCl2 dissolves in water to give the purple hexaaquo ion [V(H2O)6]2+. Evaporation of such solutions produces crystals of [V(H2O)6]Cl2.[3]
Vanadium dichloride is used as a specialty reductant in organic chemistry. As an aqueous solution, it converts cyclohexylnitrate to cyclohexanone. It reduces phenyl azide into aniline.[4]
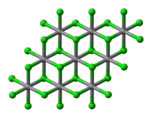
Structure
Solid VCl2 adopts the cadmium iodide structure, featuring octahedral coordination geometry. VBr2 and VI2 are structurally and chemically similar to the dichloride. All have the d3 configuration, with a quartet ground state, akin to Cr(III).[5]
References
- ↑ "Vanadium dichloride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/66355#section=Safety-and-Hazards.
- ↑ 2.0 2.1 Young, R. C.; Smith, M. E. "Vanadium(II) Chloride" Inorganic Syntheses, 1953, volume IV, page 126-127.doi:10.1002/9780470132357.ch42
- ↑ Martin Pomerantz, Gerald L. Combs, N. L. Dassanayake, "Vanadium Dichloride Solution" Inorganic Syntheses, 1982, vol. XXI, pp. 185–187. doi:10.1002/9780470132524.ch42
- ↑ Vanasse, Benoit; O'Brien, Michael K. (2001). "Vanadium(II) Chloride". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rv002. ISBN 0471936235.
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN:0-12-352651-5.
 |