Chemistry:Osmium(V) chloride
From HandWiki
| |
| Names | |
|---|---|
| Other names
pentachloroosmium, osmium pentachloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| Cl5Os | |
| Molar mass | 367.48 g·mol−1 |
| Appearance | black solid |
| Density | 4.09 |
| Melting point | 160 °C (320 °F; 433 K) |
| soluble | |
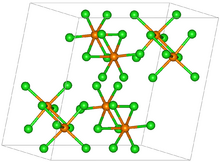
| Structure | |
| P21/c[1] | |
a = 9.17 Å, b = 11.5 Å, c = 11.97 Å α = 90°, β = 109°°, γ = 90°.[2]
| |
Lattice volume (V)
|
1194 Å3 |
Formula units (Z)
|
8 |
| Related compounds | |
Related compounds
|
Osmium pentafluoride; Rhenium pentachloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Osmium(V) chloride is an inorganic chemical compound of osmium metal and chlorine with the chemical formula OsCl
5.[3][4]
Potential synthesis
Osmium(V) chloride can be obtained in small amounts by reacting osmium hexafluoride with excess boron trichloride:[5]
- 2OsF
6 + 4BCl
3 → 2OsCl
5 + 4BF
3 + Cl
2
- 2OsF
It can also be obtained by the action of sulfur dichloride on osmium tetroxide:[2]
- 2OsO
4 + 8SCl
2 + 5Cl
2 → 2OsCl
5 + 8SOCl
2
- 2OsO
Physical properties
Osmium(V) chloride forms a black dimeric solid, isomorphic with rhenium(V) chloride. OsCl
5 is sparingly soluble in non-polar solvents.[2] It is soluble in POCl3 to form a red-brown solution, which can crystallize OsCl5·POCl3.[6]
References
- ↑ "Crystallography Open Database" (in en). qiserver.ugr.es. http://qiserver.ugr.es/cod/4344201.html.
- ↑ 2.0 2.1 2.2 Burns, Robert C.; O'Donnell, Thomas A. (1 November 1979). "Preparation and characterization of osmium pentachloride, a new binary chloride of osmium" (in en). Inorganic Chemistry 18 (11): 3081–3086. doi:10.1021/ic50201a027. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic50201a027. Retrieved 31 March 2023.
- ↑ Holleman, A. F.; Wiberg, Egon; Wiberg, Nils (2001) (in en). Inorganic Chemistry. Academic Press. p. 1461. ISBN 978-0-12-352651-9. https://books.google.com/books?id=Mtth5g59dEIC&dq=Osmium+pentachloride&pg=PA1846. Retrieved 31 March 2023.
- ↑ Dehnicke, Kurt; Lößberg, Rainer (1 December 1980). "Eine neue Synthese und das IR-Spektrum von Osmiumpentachlorid / A New Synthesis and the IR Spectrum of Osmiumpentachloride" (in de). Zeitschrift für Naturforschung B 35 (12): 1525–1528. doi:10.1515/znb-1980-1207.
- ↑ Macintyre, Jane E. (23 July 1992) (in en). Dictionary of Inorganic Compounds. CRC Press. p. 2985. ISBN 978-0-412-30120-9. https://books.google.com/books?id=9eJvoNCSCRMC&dq=Osmium+pentachloride&pg=PA2985. Retrieved 31 March 2023.
- ↑ Dehnicke, Kurt; Lößberg, Rainer (1980-12-01). "Eine neue Synthese und das IR-Spektrum von Osmiumpentachlorid / A New Synthesis and the IR Spectrum of Osmiumpentachloride". Zeitschrift für Naturforschung B (Walter de Gruyter GmbH) 35 (12): 1525–1528. doi:10.1515/znb-1980-1207. ISSN 1865-7117.
 |

