Chemistry:Hydrogen telluride
 Liquid hydrogen telluride in a test tube
| |
| |
 Tellurium, Te Hydrogen, H | |
| Names | |
|---|---|
| IUPAC name
hydrogen telluride
| |
| Other names
hydrotelluric acid
tellane tellurium hydride dihydrogen telluride tellurane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| H2Te | |
| Molar mass | 129.6158 g mol−1 |
| Appearance | colourless gas |
| Odor | Pungent, resembles rotting garlic or leeks |
| Density | 3.310 g/L, gas 2.57 g/cm3 (−20 °C, liquid) |
| Melting point | −49 °C (−56 °F; 224 K)[1] |
| Boiling point | −2.2 °C (28.0 °F; 270.9 K) (unstable above −2 °C) |
| 0.70 g/100 mL | |
| Acidity (pKa) | 2.6 |
| Conjugate acid | Telluronium |
| Conjugate base | Telluride |
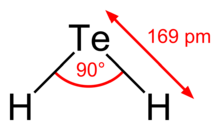
| Structure | |
| bent | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
0.7684 kJ/g |
| Hazards | |
| Main hazards | toxic |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
H2O H2S H2Se H2Po |
Other cations
|
Na2Te Ag2Te K2Te Rb2Te Cs2Te |
Related compounds
|
telluric acid tellurous acid stibine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hydrogen telluride is the inorganic compound with the formula H2Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist at very low concentrations long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.[2]
Synthesis
Electrolytic methods have been developed.[3]
H2Te can also be prepared by hydrolysis of the telluride derivatives of electropositive metals.[4] The typical hydrolysis is that of aluminium telluride:
- Al2Te3 + 6 H2O → 2 Al(OH)3 + 3 H2Te
Other salts of Te2− such as MgTe and sodium telluride can also be used. Na2Te can be made by the reaction of Na and Te in anhydrous ammonia.[5] The intermediate in the hydrolysis, HTe−, can be isolated as salts as well. NaHTe can be made by reducing tellurium with NaBH4.[5]
Hydrogen telluride cannot be efficiently prepared from its constituent elements, in contrast to H2Se.[3]
Properties
H2Te is an endothermic compound, degrading to the elements at room temperature:
- H2Te → H2 + Te
Light accelerates the decomposition. It is unstable in air, being oxidized to water and elemental tellurium:[6]
- 2 H2Te + O2 → 2 H2O + 2 Te
It is almost as acidic as phosphoric acid (Ka = 8.1×10−3), having a Ka value of about 2.3×10−3.[6] It reacts with many metals to form tellurides.[7]
See also
References
- ↑ Lide, David R., ed (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ↑ Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN:0-7506-3365-4.
- ↑ 3.0 3.1 F. Fehér, "Hydrogen Telluride" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. pp. 438.
- ↑ Shriver, Atkins. Inorganic Chemistry, Fifth Edition. W. H. Freeman and Company, New York, 2010; pp 407.
- ↑ 5.0 5.1 Nicola Petragnani; Hélio A. Stefani (2007). Tellurium in organic synthesis. Best synthetic methods (2nd ed.). Academic Press. p. 6. ISBN 978-0-08-045310-1.
- ↑ 6.0 6.1 Egon Wiberg; Arnold Frederick Holleman (2001). Nils Wiberg. ed. Inorganic chemistry. Academic Press. p. 589. ISBN 0-12-352651-5.
- ↑ Henry Enfield Roscoe; Carl Schorlemmer (1878). A treatise on chemistry. 1. Appleton. pp. 367–368.
 |









