Chemistry:Leucine
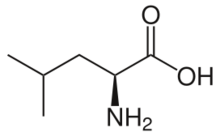 Skeletal formula of L-leucine
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Leucine
| |||
| Other names
2-Amino-4-methylpentanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H13NO2 | |||
| Molar mass | 131.175 g·mol−1 | ||
| Acidity (pKa) | 2.36 (carboxyl), 9.60 (amino)[2] | ||
| -84.9·10−6 cm3/mol | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Leucine (symbol Leu or L)[3] is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG.
Like valine and isoleucine, leucine is a branched-chain amino acid. The primary metabolic end products of leucine metabolism are acetyl-CoA and acetoacetate; consequently, it is one of the two exclusively ketogenic amino acids, with lysine being the other.[4] It is the most important ketogenic amino acid in humans.[5]
Leucine and β-hydroxy β-methylbutyric acid, a minor leucine metabolite, exhibit pharmacological activity in humans and have been demonstrated to promote protein biosynthesis via the phosphorylation of the mechanistic target of rapamycin (mTOR).[6][7]
Dietary leucine
As a food additive, L-leucine has E number E641 and is classified as a flavor enhancer.[8]
Requirements
The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For leucine, for adults 19 years and older, 42 mg/kg body weight/day.[9]
Sources
| Food | g/100g |
|---|---|
| Whey protein concentrate, dry powder | 10.0-12.0 |
| Soy protein concentrate, dry powder | 7.5-8.5 |
| Pea protein concentrate, dry powder | 6.6 |
| Soybeans, mature seeds, roasted, salted | 2.87 |
| Hemp seed, hulled | 2.16 |
| Beef, round, top round, raw | 1.76 |
| Peanuts | 1.67 |
| Fish, salmon, pink, raw | 1.62 |
| Wheat germ | 1.57 |
| Almonds | 1.49 |
| Chicken, broilers or fryers, thigh, raw | 1.48 |
| Chicken egg, yolk, raw | 1.40 |
| Oats | 1.28 |
| Edamame (soybeans, green, raw) | 0.93 |
| Beans, pinto, cooked | 0.78 |
| Lentils, cooked | 0.65 |
| Chickpea, cooked | 0.63 |
| Corn, yellow | 0.35 |
| Cow milk, whole, 3.25% milk fat | 0.27 |
| Rice, brown, medium-grain, cooked | 0.19 |
| Milk, human, mature, fluid | 0.10 |
Health effects
As a dietary supplement, leucine has been found to slow the degradation of muscle tissue by increasing the synthesis of muscle proteins in aged rats.[11] However, results of comparative studies are conflicted. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men.[12] More studies are needed, preferably ones based on an objective, random sample of society. Factors such as lifestyle choices, age, gender, diet, exercise, etc. must be factored into the analyses to isolate the effects of supplemental leucine as a standalone, or if taken with other branched chain amino acids (BCAAs). Until then, dietary supplemental leucine cannot be associated as the prime reason for muscular growth or optimal maintenance for the entire population.
Both L-leucine and D-leucine protect mice against epileptic seizures.[13] D-leucine also terminates seizures in mice after the onset of seizure activity, at least as effectively as diazepam and without sedative effects.[13] Decreased dietary intake of L-leucine lessens adiposity in mice.[14] High blood levels of leucine are associated with insulin resistance in humans, mice, and rodents.[15] This might be due to the effect of leucine to stimulate mTOR signaling.[16] Dietary restriction of leucine and the other BCAAs can reverse diet-induced obesity in wild-type mice by increasing energy expenditure, and can restrict fat mass gain of hyperphagic rats.[17][18]
Safety
Leucine toxicity, as seen in decompensated maple syrup urine disease, causes delirium and neurologic compromise, and can be life-threatening.[19]
A high intake of leucine may cause or exacerbate symptoms of pellagra in people with low niacin status because it interferes with the conversion of L-tryptophan to niacin.[20]
Leucine at a dose exceeding 500 mg/kg/d was observed with hyperammonemia.[21] As such, unofficially, a tolerable upper intake level (UL) for leucine in healthy adult men can be suggested at 500 mg/kg/d or 35 g/d under acute dietary conditions.[21][22]
Pharmacology
Pharmacodynamics
Leucine is a dietary amino acid with the capacity to directly stimulate myofibrillar muscle protein synthesis.[23] This effect of leucine results from its role as an activator of the mechanistic target of rapamycin (mTOR),[7] a serine-threonine protein kinase that regulates protein biosynthesis and cell growth. The activation of mTOR by leucine is mediated through Rag GTPases,[24][25][26] leucine binding to leucyl-tRNA synthetase,[24][25] leucine binding to sestrin 2,[27][28][29] and possibly other mechanisms.
Metabolism in humans
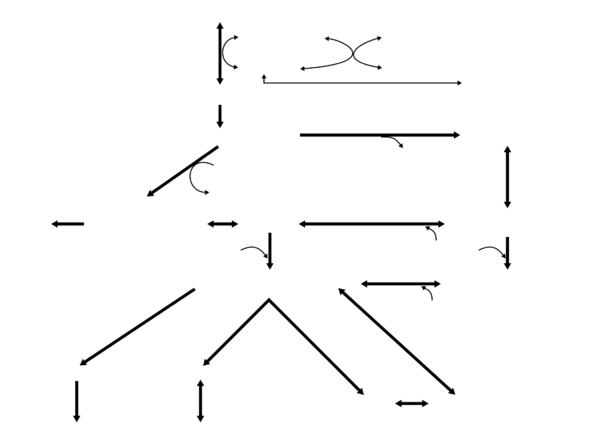
Leucine metabolism in humans
|
Leucine metabolism occurs in many tissues in the human body; however, most dietary leucine is metabolized within the liver, adipose tissue, and muscle tissue.[35] Adipose and muscle tissue use leucine in the formation of sterols and other compounds.[35] Combined leucine use in these two tissues is seven times greater than in the liver.[35]
{{#Section-h:Beta-Hydroxy beta-methylbutyric acid|Biosynthesis}}
A small fraction of L-leucine metabolism – less than 5% in all tissues except the testes where it accounts for about 33% – is initially catalyzed by leucine aminomutase, producing β-leucine, which is subsequently metabolized into β-ketoisocaproate (β-KIC), β-ketoisocaproyl-CoA, and then acetyl-CoA by a series of uncharacterized enzymes.[34][36]
{{#Section-h:Beta-Hydroxy beta-methylbutyric acid|Metabolism}}
Synthesis in non-human organisms
Leucine is an essential amino acid in the diet of animals because they lack the complete enzyme pathway to synthesize it de novo from potential precursor compounds. Consequently, they must ingest it, usually as a component of proteins. Plants and microorganisms synthesize leucine from pyruvic acid with a series of enzymes:[37]
- Acetolactate synthase
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- α-Isopropylmalate synthase
- α-Isopropylmalate isomerase
- Leucine aminotransferase
Synthesis of the small, hydrophobic amino acid valine also includes the initial part of this pathway.
Chemistry
Leucine is a branched-chain amino acid (BCAA) since it possesses an aliphatic side-chain that is not linear.
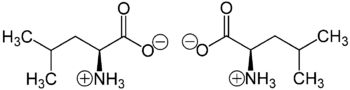
Racemic leucine had been[when?] subjected to circularly polarized synchrotron radiation to better understand the origin of biomolecular asymmetry. An enantiomeric enhancement of 2.6% had been induced, indicating a possible photochemical origin of biomolecules' homochirality.[38]
See also
- Leucines, the isomers and derivatives of leucine
- Leucine zipper, a common motif in transcription factor proteins
Notes
- ↑ This reaction is catalyzed by an unknown thioesterase enzyme.[30][31]
References
- ↑ 1.0 1.1 "Accurate hydrogen parameters for the amino acid L-leucine". Acta Crystallographica Section B 72 (Pt 6): 885–892. December 2016. doi:10.1107/S2052520616015699. PMID 27910839. https://www.pure.ed.ac.uk/ws/files/36568495/20170523_Parsons_Leucine_0410.pdf.
- ↑ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ↑ "Nomenclature and Symbolism for Amino Acids and Peptides". IUPAC-IUB Joint Commission on Biochemical Nomenclature. 1983. http://www.chem.qmul.ac.uk/iupac/AminoAcid/AA1n2.html.
- ↑ (in en) Biochemistry. Lippincott Williams & Wilkins. 2013-05-24. ISBN 9781451175622. https://books.google.com/books?id=sSyMAwAAQBAJ&pg=PA266.
- ↑ (in en) Metabolic & Therapeutic Aspects of Amino Acids in Clinical Nutrition (Second ed.). CRC Press. 2003-11-13. p. 101. ISBN 9780203010266. https://books.google.com/books?id=X3DK4nWybaMC.
- ↑ "β-hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: a systematic review". Nutrition Research 45: 1–9. September 2017. doi:10.1016/j.nutres.2017.07.008. PMID 29037326. "HMB's mechanisms of action are generally considered to relate to its effect on both muscle protein synthesis and muscle protein breakdown (Figure 1) [2, 3]. HMB appears to stimulate muscle protein synthesis through an up-regulation of the mammalian/mechanistic target of rapamycin complex 1 (mTORC1), a signaling cascade involved in coordination of translation initiation of muscle protein synthesis [2, 4]. Additionally, HMB may have antagonistic effects on the ubiquitin–proteasome pathway, a system that degrades intracellular proteins [5, 6]. Evidence also suggests that HMB promotes myogenic proliferation, differentiation, and cell fusion [7]. ... Exogenous HMB-FA administration has shown to increase intramuscular anabolic signaling, stimulate muscle protein synthesis, and attenuate muscle protein breakdown in humans [2].".
- ↑ 7.0 7.1 "Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism". The Journal of Physiology 591 (11): 2911–23. June 2013. doi:10.1113/jphysiol.2013.253203. PMID 23551944. "The stimulation of MPS through mTORc1-signalling following HMB exposure is in agreement with pre-clinical studies (Eley et al. 2008). ... Furthermore, there was clear divergence in the amplitude of phosphorylation for 4EBP1 (at Thr37/46 and Ser65/Thr70) and p70S6K (Thr389) in response to both Leu and HMB, with the latter showing more pronounced and sustained phosphorylation. ... Nonetheless, as the overall MPS response was similar, this cellular signalling distinction did not translate into statistically distinguishable anabolic effects in our primary outcome measure of MPS. ... Interestingly, although orally supplied HMB produced no increase in plasma insulin, it caused a depression in MPB (−57%). Normally, postprandial decreases in MPB (of ~50%) are attributed to the nitrogen-sparing effects of insulin since clamping insulin at post-absorptive concentrations (5 μU ml−1) while continuously infusing AAs (18 g h−1) did not suppress MPB (Greenhaff et al. 2008), which is why we chose not to measure MPB in the Leu group, due to an anticipated hyperinsulinaemia (Fig. 3C). Thus, HMB reduces MPB in a fashion similar to, but independent of, insulin. These findings are in-line with reports of the anti-catabolic effects of HMB suppressing MPB in pre-clinical models, via attenuating proteasomal-mediated proteolysis in response to LPS (Eley et al. 2008).".
- ↑ A consumer's dictionary of food additives (7th ed.). New York: Three Rivers Press. 2009. ISBN 978-0307408921. https://archive.org/details/isbn_9780307408921.
- ↑ Institute of Medicine (2002). "Protein and Amino Acids". Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington, DC: The National Academies Press. pp. 589–768. doi:10.17226/10490. ISBN 978-0-309-08525-0. https://www.nap.edu/read/10490/chapter/12.
- ↑ National Nutrient Database for Standard Reference. U.S. Department of Agriculture. http://www.nal.usda.gov/fnic/foodcomp/search/. Retrieved 16 September 2009.
- ↑ "A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle". The Journal of Physiology 569 (Pt 2): 489–99. December 2005. doi:10.1113/jphysiol.2005.098004. PMID 16195315.
- ↑ "Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men". The American Journal of Clinical Nutrition 89 (5): 1468–75. May 2009. doi:10.3945/ajcn.2008.26668. PMID 19321567.
- ↑ 13.0 13.1 "Potent anti-seizure effects of D-leucine". Neurobiology of Disease 82: 46–53. October 2015. doi:10.1016/j.nbd.2015.05.013. PMID 26054437.
- ↑ "Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health". Cell Reports 16 (2): 520–530. July 2016. doi:10.1016/j.celrep.2016.05.092. PMID 27346343.
- ↑ "Branched-chain amino acids in metabolic signalling and insulin resistance". Nature Reviews. Endocrinology 10 (12): 723–36. December 2014. doi:10.1038/nrendo.2014.171. PMID 25287287.
- ↑ "The Roles of mTOR Complexes in Lipid Metabolism". Annual Review of Nutrition 35: 321–48. 2015. doi:10.1146/annurev-nutr-071714-034355. PMID 26185979.
- ↑ "Restoration of metabolic health by decreased consumption of branched-chain amino acids". The Journal of Physiology 596 (4): 623–645. February 2018. doi:10.1113/JP275075. PMID 29266268.
- ↑ "Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export". Molecular Metabolism 5 (7): 538–551. July 2016. doi:10.1016/j.molmet.2016.04.006. PMID 27408778.
- ↑ Yudkoff, Marc; Daikhin, Yevgeny; Nissim, Ilana; Horyn, Oksana; Luhovyy, Bohdan; Lazarow, Adam; Nissim, Itzhak (2005-06-01). "Brain Amino Acid Requirements and Toxicity: The Example of Leucine" (in en). The Journal of Nutrition 135 (6): 1531S–1538S. doi:10.1093/jn/135.6.1531S. ISSN 0022-3166. PMID 15930465. https://academic.oup.com/jn/article/135/6/1531S/4663840.
- ↑ "Mechanisms of the pellagragenic effect of leucine: stimulation of hepatic tryptophan oxidation by administration of branched-chain amino acids to healthy human volunteers and the role of plasma free tryptophan and total kynurenines". International Journal of Tryptophan Research 7: 23–32. 2014. doi:10.4137/IJTR.S18231. PMID 25520560.
- ↑ 21.0 21.1 "Determination of the tolerable upper intake level of leucine in acute dietary studies in young men". The American Journal of Clinical Nutrition 96 (4): 759–67. October 2012. doi:10.3945/ajcn.111.024471. PMID 22952178. "A significant increase in blood ammonia concentrations above normal values, plasma leucine concentrations, and urinary leucine excretion were observed with leucine intakes >500 mg · kg−1 · d−1. The oxidation of l-[1-13C]-leucine expressed as label tracer oxidation in breath (F13CO2), leucine oxidation, and α-ketoisocaproic acid (KIC) oxidation led to different results: a plateau in F13CO2 observed after 500 mg · kg−1 · d−1, no clear plateau observed in leucine oxidation, and KIC oxidation appearing to plateau after 750 mg · kg−1 · d−1. On the basis of plasma and urinary variables, the UL for leucine in healthy adult men can be suggested at 500 mg · kg−1 · d−1 or ~35 g/d as a cautious estimate under acute dietary conditions.".
- ↑ "Determination of the safety of leucine supplementation in healthy elderly men". Amino Acids 48 (7): 1707–16. July 2016. doi:10.1007/s00726-016-2241-0. PMID 27138628. "the upper limit for leucine intake in healthy elderly could be set similar to young men at 500 mg kg-1 day-1 or ~35 g/day for an individual weighing 70 kg".
- ↑ "Manufacture and use of dairy protein fractions". The Journal of Nutrition 134 (4): 996S–1002S. April 2004. doi:10.1093/jn/134.4.996S. PMID 15051860.
- ↑ 24.0 24.1 "Control of leucine-dependent mTORC1 pathway through chemical intervention of leucyl-tRNA synthetase and RagD interaction". Nature Communications 8 (1): 732. September 2017. doi:10.1038/s41467-017-00785-0. PMID 28963468. Bibcode: 2017NatCo...8..732K.
- ↑ 25.0 25.1 "Amino acid signalling upstream of mTOR". Nature Reviews. Molecular Cell Biology 14 (3): 133–9. March 2013. doi:10.1038/nrm3522. PMID 23361334.
- ↑ "The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1". Science 320 (5882): 1496–501. June 2008. doi:10.1126/science.1157535. PMID 18497260. Bibcode: 2008Sci...320.1496S.
- ↑ "Sestrin2 is a leucine sensor for the mTORC1 pathway". Science 351 (6268): 43–8. January 2016. doi:10.1126/science.aab2674. PMID 26449471. Bibcode: 2016Sci...351...43W.
- ↑ "Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway". Science 351 (6268): 53–8. January 2016. doi:10.1126/science.aad2087. PMID 26586190. Bibcode: 2016Sci...351...53S.
- ↑ "The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1". Cell Reports 9 (1): 1–8. October 2014. doi:10.1016/j.celrep.2014.09.014. PMID 25263562.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedHMB-CoA ⇔ HMB - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid21918059 - ↑ 32.0 32.1 "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". Journal of the International Society of Sports Nutrition 10 (1): 6. February 2013. doi:10.1186/1550-2783-10-6. PMID 23374455.
- ↑ 34.0 34.1 34.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedLeucine metabolism - ↑ 35.0 35.1 35.2 "Metabolic fate of leucine: a significant sterol precursor in adipose tissue and muscle". The American Journal of Physiology 226 (2): 411–8. February 1974. doi:10.1152/ajplegacy.1974.226.2.411. PMID 4855772.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBRENDA leucine metabolism - ↑ Lehninger principles of biochemistry. (3rd ed.). New York: Worth Publishers. 2000. ISBN 978-1-57259-153-0.
- ↑ Meierhenrich: Amino acids and the asymmetry of life, Springer-Verlag, 2008, ISBN:978-3-540-76885-2.
External links
 |