Chemistry:Hydrogen cyanide

| |||
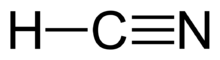
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name | |||
| Systematic IUPAC name
Hydrogen cyanide (gas form) Hydrocyanic acid (aqueous) | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
| MeSH | Hydrogen+Cyanide | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1051 | ||
| |||
| |||
| Properties | |||
| HCN | |||
| Molar mass | 27.0253 g/mol | ||
| Appearance | Colorless liquid or gas | ||
| Odor | Almond-like[3] | ||
| Melting point | −13.29 °C (8.08 °F; 259.86 K)[4] | ||
| Boiling point | 26 °C (79 °F; 299 K)[4]:4.67 | ||
| Miscible | |||
| Solubility in ethanol | Miscible | ||
| Vapor pressure | 100 kPa (25 °C)[4]:6.94 | ||
Henry's law
constant (kH) |
75 μmol Pa−1 kg−1 | ||
| Acidity (pKa) | 9.21 (in water),
12.9 (in DMSO)[5] | ||
| Basicity (pKb) | 4.79 (cyanide anion) | ||
| Conjugate acid | Hydrocyanonium | ||
| Conjugate base | Cyanide | ||
Refractive index (nD)
|
1.2675[6] | ||
| Viscosity | 0.183 mPa·s (25 °C)[4]:6.231 | ||
| Structure | |||
| tetragonal (>170 K) orthorhombic (<170 K)[7] | |||
| C∞v | |||
| Linear | |||
| 2.98 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
35.9 J K−1 mol−1 (gas)[4]:5.19 | ||
Std molar
entropy (S |
201.8 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
135.1 kJ mol−1 | ||
| Hazards | |||
| GHS pictograms |    
| ||
| GHS Signal word | Danger | ||
| H225, H300, H310, H319, H330, H336, H370, H410 | |||
| P210, P261, P305+351+338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −17.8 °C (0.0 °F; 255.3 K) | ||
| 538 °C (1,000 °F; 811 K) | |||
| Explosive limits | 5.6% – 40.0%[8] | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration)
|
501 ppm (rat, 5 min) 323 ppm (mouse, 5 min) 275 ppm (rat, 15 min) 170 ppm (rat, 30 min) 160 ppm (rat, 30 min) 323 ppm (rat, 5 min)[9] | ||
LCLo (lowest published)
|
200 ppm (mammal, 5 min) 36 ppm (mammal, 2 hr) 107 ppm (human, 10 min) 759 ppm (rabbit, 1 min) 759 ppm (cat, 1 min) 357 ppm (human, 2 min) 179 ppm (human, 1 hr)[9] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 10 ppm (11 mg/m3) [skin][8] | ||
REL (Recommended)
|
ST 4.7 ppm (5 mg/m3) [skin][8] | ||
IDLH (Immediate danger)
|
50 ppm[8] | ||
| Related compounds | |||
Related alkanenitriles
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hydrogen cyanide (also known as prussic acid) is a chemical compound with the formula HCN and structural formula H–C≡N. It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively.[10] It is more toxic than solid cyanide compounds due to its volatile nature.
Structure and general properties
Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide.[citation needed]
Hydrogen cyanide is weakly acidic with a pKa of 9.2. It partially ionizes in water to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.
HCN has a faint bitter almond-like odor that some people are unable to detect owing to a recessive genetic trait.[11] The volatile compound has been used as inhalation rodenticide and human poison, as well as for killing whales.[12] Cyanide ions interfere with iron-containing respiratory enzymes.[citation needed]
Chemical properties
Hydrogen cyanide will react with alkenes under catalysis of nickel complexes. This reaction is called hydrocyanation.[13]
- RCH=CH2 + HCN → RCH2-CH2-CN
Four molecules of HCN will tetramerize into diaminomaleonitrile, which can be converted to various purines.[14]
History of discovery

Hydrogen cyanide was first isolated from a blue pigment (Prussian blue) which had been known since 1706, but whose structure was unknown. It is now known to be a coordination polymer with a complex structure and an empirical formula of hydrated ferric ferrocyanide. In 1752, the French chemist Pierre Macquer made the important step of showing that Prussian blue could be converted to an iron oxide plus a volatile component and that these could be used to reconstitute it.[15] The new component was what is now known as hydrogen cyanide. Following Macquer's lead, it was first prepared from Prussian blue by the Swedish chemist Carl Wilhelm Scheele in 1782,[16] and was eventually given the German name Blausäure (lit. "Blue acid") because of its acidic nature in water and its derivation from Prussian blue. In English, it became known popularly as prussic acid.
In 1787, the French chemist Claude Louis Berthollet showed that prussic acid did not contain oxygen,[17] an important contribution to acid theory, which had hitherto postulated that acids must contain oxygen[18] (hence the name of oxygen itself, which is derived from Greek elements that mean "acid-former" and are likewise calqued into German as Sauerstoff). In 1811, Joseph Louis Gay-Lussac prepared pure, liquified hydrogen cyanide.[19] In 1815, Gay-Lussac deduced Prussic acid's chemical formula.[20] The radical cyanide in hydrogen cyanide was given its name from cyan, not only an English word for a shade of blue but the Greek word for blue (Ancient Greek:), again owing to its derivation from Prussian blue.
Production and synthesis
Hydrogen cyanide forms in at least limited amounts from many combinations of hydrogen, carbon, and ammonia. Hydrogen cyanide is produced in large quantities by several processes and is a recovered waste product from the manufacture of acrylonitrile.[10] In 2006, between 500 million and 1 billion pounds (between 230,000 and 450,000 t) were produced in the US.[21]
The most important process is the Andrussow oxidation invented by Leonid Andrussow at IG Farben in which methane and ammonia react in the presence of oxygen at about 1,200 °C (2,190 °F) over a platinum catalyst:[22]
- 2 CH4 + 2 NH3 + 3 O2 → 2 HCN + 6 H2O
The energy needed for the reaction is provided by the partial oxidation of methane and ammonia.
Of lesser importance is the Degussa process (BMA process) in which no oxygen is added and the energy must be transferred indirectly through the reactor wall:[23]
- CH4 + NH3 → HCN + 3H2
This reaction is akin to steam reforming, the reaction of methane and water to give carbon monoxide and hydrogen.
In the Shawinigan Process, hydrocarbons, e.g. propane, are reacted with ammonia.
In the laboratory, small amounts of HCN are produced by the addition of acids to cyanide salts of alkali metals:
- H+ + NaCN → HCN + Na+
This reaction is sometimes the basis of accidental poisonings because the acid converts a nonvolatile cyanide salt into the gaseous HCN.
Hydrogen cyanide could be obtained from potassium ferricyanide and acid:
Historical methods of production
The large demand for cyanides for mining operations in the 1890s was met by George Thomas Beilby, who patented a method to produce hydrogen cyanide by passing ammonia over glowing coal in 1892. This method was used until Hamilton Castner in 1894 developed a synthesis starting from coal, ammonia, and sodium yielding sodium cyanide, which reacts with acid to form gaseous HCN.
Applications
HCN is the precursor to sodium cyanide and potassium cyanide, which are used mainly in gold and silver mining and for the electroplating of those metals. Via the intermediacy of cyanohydrins, a variety of useful organic compounds are prepared from HCN including the monomer methyl methacrylate, from acetone, the amino acid methionine, via the Strecker synthesis, and the chelating agents EDTA and NTA. Via the hydrocyanation process, HCN is added to butadiene to give adiponitrile, a precursor to Nylon-6,6.[10]
HCN is used globally as a fumigant against many species of pest insects that infest food production facilities. Both its efficacy and method of application lead to very small amounts of the fumigant being used compared to other toxic substances used for the same purpose.[26] Using HCN as a fumigant also has minimal environmental impact, compared to similar structural fumigant molecules such as sulfuryl fluoride,[27] and methyl bromide.[28]
Occurrence
HCN is obtainable from fruits that have a pit, such as cherries, apricots, apples, and bitter almonds, from which almond oil and flavoring are made. Many of these pits contain small amounts of cyanohydrins such as mandelonitrile and amygdalin, which slowly release hydrogen cyanide.[29][30] One hundred grams of crushed apple seeds can yield about 70 mg of HCN.[31] So-called "bitter" roots of the cassava plant may contain up to 1 gram of HCN per kilogram.[32][33] Some millipedes, such as Harpaphe haydeniana, Desmoxytes purpurosea, and Apheloria release hydrogen cyanide as a defense mechanism,[34] as do certain insects, such as burnet moths and the larvae of Paropsisterna eucalyptus.[35] Hydrogen cyanide is contained in the exhaust of vehicles, and in smoke from burning nitrogen-containing plastics.
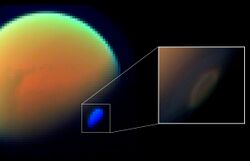
On Titan
HCN has been measured in Titan's atmosphere by four instruments on the Cassini space probe, one instrument on Voyager, and one instrument on Earth.[36] One of these measurements was in situ, where the Cassini spacecraft dipped between 1,000 and 1,100 km (620 and 680 mi) above Titan's surface to collect atmospheric gas for mass spectrometry analysis.[37] HCN initially forms in Titan's atmosphere through the reaction of photochemically produced methane and nitrogen radicals which proceed through the H2CN intermediate, e.g., (CH3 + N → H2CN + H → HCN + H2).[38][39] Ultraviolet radiation breaks HCN up into CN + H; however, CN is efficiently recycled back into HCN via the reaction CN + CH4 → HCN + CH3.[38]
On the young Earth
It has been postulated that carbon from a cascade of asteroids (known as the Late Heavy Bombardment), resulting from interaction of Jupiter and Saturn, blasted the surface of young Earth and reacted with nitrogen in Earth's atmosphere to form HCN.[40]
In mammals
Some authors[who?] have shown that neurons can produce hydrogen cyanide upon activation of their opioid receptors by endogenous or exogenous opioids. They have also shown that neuronal production of HCN activates NMDA receptors and plays a role in signal transduction between neuronal cells (neurotransmission). Moreover, increased endogenous neuronal HCN production under opioids was seemingly needed for adequate opioid analgesia, as analgesic action of opioids was attenuated by HCN scavengers. They considered endogenous HCN to be a neuromodulator.[41]
It has also been shown that, while stimulating muscarinic cholinergic receptors in cultured pheochromocytoma cells increases HCN production, in a living organism (in vivo) muscarinic cholinergic stimulation actually decreases HCN production.[42]
Leukocytes generate HCN during phagocytosis, and can kill bacteria, fungi, and other pathogens by generating several different toxic chemicals, one of which is hydrogen cyanide.[41]
The vasodilatation caused by sodium nitroprusside has been shown to be mediated not only by NO generation, but also by endogenous cyanide generation, which adds not only toxicity, but also some additional antihypertensive efficacy compared to nitroglycerine and other non-cyanogenic nitrates which do not cause blood cyanide levels to rise.[43]
HCN is a constituent of tobacco smoke.[44]
HCN and the origin of life
Hydrogen cyanide has been discussed as a precursor to amino acids and nucleic acids, and is proposed to have played a part in the origin of life.[45] Although the relationship of these chemical reactions to the origin of life theory remains speculative, studies in this area have led to discoveries of new pathways to organic compounds derived from the condensation of HCN (e.g. Adenine).[46]
In space
HCN has been detected in the interstellar medium[47] and in the atmospheres of carbon stars.[48] Since then, extensive studies have probed formation and destruction pathways of HCN in various environments and examined its use as a tracer for a variety of astronomical species and processes. HCN can be observed from ground-based telescopes through a number of atmospheric windows.[49] The J=1→0, J=3→2, J= 4→3, and J=10→9 pure rotational transitions have all been observed.[47][50][51]
HCN is formed in interstellar clouds through one of two major pathways:[52] via a neutral-neutral reaction (CH2 + N → HCN + H) and via dissociative recombination (HCNH+ + e− → HCN + H). The dissociative recombination pathway is dominant by 30%; however, the HCNH+ must be in its linear form. Dissociative recombination with its structural isomer, H2NC+, exclusively produces hydrogen isocyanide (HNC).
HCN is destroyed in interstellar clouds through a number of mechanisms depending on the location in the cloud.[52] In photon-dominated regions (PDRs), photodissociation dominates, producing CN (HCN + ν → CN + H). At further depths, photodissociation by cosmic rays dominate, producing CN (HCN + cr → CN + H). In the dark core, two competing mechanisms destroy it, forming HCN+ and HCNH+ (HCN + H+ → HCN+ + H; HCN + HCO+ → HCNH+ + CO). The reaction with HCO+ dominates by a factor of ~3.5. HCN has been used to analyze a variety of species and processes in the interstellar medium. It has been suggested as a tracer for dense molecular gas[53][54] and as a tracer of stellar inflow in high-mass star-forming regions.[55] Further, the HNC/HCN ratio has been shown to be an excellent method for distinguishing between PDRs and X-ray-dominated regions (XDRs).[56]
On 11 August 2014, astronomers released studies, using the Atacama Large Millimeter/Submillimeter Array (ALMA) for the first time, that detailed the distribution of HCN, HNC, H2CO, and dust inside the comae of comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON).[57][58]
In February 2016, it was announced that traces of hydrogen cyanide were found in the atmosphere of the hot Super-Earth 55 Cancri e with NASA's Hubble Space Telescope.[59]
On 14 December 2023, astronomers reported the first time discovery, in the plumes of Enceladus, moon of the planet Saturn, of hydrogen cyanide, a possible chemical essential for life[60] as we know it, as well as other organic molecules, some of which are yet to be better identified and understood. According to the researchers, "these [newly discovered] compounds could potentially support extant microbial communities or drive complex organic synthesis leading to the origin of life."[61][62]
As a poison and chemical weapon
In World War I, hydrogen cyanide was used by the French from 1916 as a chemical weapon against the Central Powers, and by the United States and Italy in 1918. It was not found to be effective enough due to weather conditions.[63][64] The gas is lighter than air and rapidly disperses up into the atmosphere. Rapid dilution made its use in the field impractical. In contrast, denser agents such as phosgene or chlorine tended to remain at ground level and sank into the trenches of the Western Front's battlefields. Compared to such agents, hydrogen cyanide had to be present in higher concentrations in order to be fatal.
A hydrogen cyanide concentration of 100–200 ppm in breathing air will kill a human within 10 to 60 minutes.[65] A hydrogen cyanide concentration of 2000 ppm (about 2380 mg/m3) will kill a human in about one minute.[65] The toxic effect is caused by the action of the cyanide ion, which halts cellular respiration. It acts as a non-competitive inhibitor for an enzyme in mitochondria called cytochrome c oxidase. As such, hydrogen cyanide is commonly listed among chemical weapons as a blood agent.[66]
The Chemical Weapons Convention lists it under Schedule 3 as a potential weapon which has large-scale industrial uses. Signatory countries must declare manufacturing plants that produce more than 30 metric tons per year, and allow inspection by the Organisation for the Prohibition of Chemical Weapons.
Perhaps its most infamous use is Zyklon B (German: Cyclone B, with the B standing for Blausäure – prussic acid; also, to distinguish it from an earlier product later known as Zyklon A),[67] used in Nazi German extermination camps during World War II to kill en masse as part of their Final Solution genocide program. Hydrogen cyanide was also used in the camps for delousing clothing in attempts to eradicate diseases carried by lice and other parasites. One of the original Czech producers continued making Zyklon B under the trademark "Uragan D2"[68] until around 2015.[69]
During World War II, the US considered using it, along with cyanogen chloride, as part of Operation Downfall, the planned invasion of Japan, but President Harry Truman decided against it, instead using the atomic bombs developed by the secret Manhattan Project.[70]
Hydrogen cyanide was also the agent employed in judicial execution in some U.S. states, where it was produced during the execution by the action of sulfuric acid on sodium or potassium cyanide.[71]
Under the name prussic acid, HCN has been used as a killing agent in whaling harpoons, although it proved quite dangerous to the crew deploying it, and it was quickly abandoned.[12] From the middle of the 18th century it was used in a number of poisoning murders and suicides.[72]
Hydrogen cyanide gas in air is explosive at concentrations above 5.6%.[73]
References
- ↑ "Hydrogen Cyanide – Compound Summary". PubChem Compound. United States: National Center for Biotechnology Information. 16 September 2004. Identification. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=768.
- ↑ "hydrogen cyanide (CHEBI:18407)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 18 October 2009. Main. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=18407.
- ↑ https://apps.who.int/iris/rest/bitstreams/50961/retrieve
- ↑ 4.0 4.1 4.2 4.3 4.4 Cite error: Invalid
<ref>tag; no text was provided for refs namedHaynes - ↑ "pKa's of Inorganic and Oxo-Acids". http://ccc.chem.pitt.edu/wipf/MechOMs/evans_pKa_table.pdf.
- ↑ Handbook of Inorganic Chemicals. McGraw-Hill. 2002. ISBN 978-0070494398.
- ↑ Schulz, Axel; Surkau, Jonas (2022-09-21). "Main group cyanides: from hydrogen cyanide to cyanido-complexes". Reviews in Inorganic Chemistry (Walter de Gruyter GmbH) 43 (1): 49–188. doi:10.1515/revic-2021-0044. ISSN 0193-4929.
- ↑ 8.0 8.1 8.2 8.3 NIOSH Pocket Guide to Chemical Hazards. "#0333". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0333.html.
- ↑ 9.0 9.1 "Hydrogen cyanide". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/74908.html.
- ↑ 10.0 10.1 10.2 Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_159.pub2.
- ↑ "Cyanide, inability to smell". Online Mendelian Inheritance in Man. https://www.ncbi.nlm.nih.gov/omim/304300.
- ↑ 12.0 12.1 "Poison Harpoons". http://www.whalecraft.net/Poison_Irons.html.
- ↑ Leeuwen, P. W. N. M. van (2004). Homogeneous catalysis : understanding the art. Dordrecht: Kluwer Academic Publishers. ISBN 1402019998. OCLC 54966334.
- ↑ Bray, D; Robbins, P (1967). "Mechanism of ε15 conversion studied with bacteriophage mutants". Journal of Molecular Biology (Elsevier BV) 30 (2): 457–475. doi:10.1016/s0022-2836(67)80037-8. ISSN 0022-2836.
- ↑ "Éxamen chymique de bleu de Prusse" (in French). Mémoires de l'Académie royale des Sciences: 60–77. 1756. http://gallica.bnf.fr/ark:/12148/bpt6k35505/f242.
- ↑ "Försök, beträffande det färgande ämnet uti Berlinerblå" (in Swedish). Kungliga Svenska Vetenskapsakademiens Handlingar (Royal Swedish Academy of Science's Proceedings 3: 264–275. 1782. https://books.google.com/books?id=mHVJAAAAcAAJ&pg=PA264.
Reprinted in Latin as: "De materia tingente caerulei berolinensis" (in Latin). Opuscula Chemica et Physica. 2. (Leipzig ("Lipsiae") (Germany): Johann Godfried Müller. 1789. pp. 148–174. https://books.google.com/books?id=BLo5AAAAcAAJ&pg=PA148. - ↑ "Mémoire sur l'acide prussique" (in French). Mémoires de l'Académie Royale des Sciences: 148–161. 1789. https://books.google.com/books?id=fC5EAAAAcAAJ&pg=PA148.
Reprinted in: "Extrait d'un mémoire sur l'acide prussique". Annales de Chimie 1: 30–39. 1789. http://gallica.bnf.fr/ark:/12148/bpt6k110315k/f40.image.langEN. - ↑ "Claude Louis Berthollet: A Great Chemist in the French Tradition". Canadian Chemical News. 1999-11-01. http://www.allbusiness.com/north-america/canada/370855-1.html.
- ↑ "Note sur l'acide prussique". Annales de Chimie 44: 128–133. 1811. https://books.google.com/books?id=uJs5AAAAcAAJ&pg=PA128.
- ↑ "Recherche sur l'acide prussique". Annales de Chimie 95: 136–231. 1815. https://books.google.com/books?id=m9s3AAAAMAAJ&pg=PA136.
- ↑ Non-confidential 2006 IUR Records by Chemical, including Manufacturing, Processing and Use Information. EPA. Retrieved on 2013-01-31.
- ↑ "The catalytic oxydation of ammonia-methane-mixtures to hydrogen cyanide". Angewandte Chemie 48 (37): 593–595. 1935. doi:10.1002/ange.19350483702. Bibcode: 1935AngCh..48..593A.
- ↑ "Die technische Synthese von Cyanwasserstoff aus Methan und Ammoniak ohne Zusatz von Sauerstoff". Chemie Ingenieur Technik 30 (5): 305–310. 1958. doi:10.1002/cite.330300506.
- ↑ "MSDS for potassium ferricyanide". http://www.labchem.com/tools/msds/msds/LC19040.pdf.
- ↑ "Potassium ferricyanide". https://pubchem.ncbi.nlm.nih.gov/compound/26250.
- ↑ "Manual of fumigation for insect control – Space fumigation at atmospheric pressure (Cont.)". http://www.fao.org/3/X5042E/x5042E0k.htm#Fumigation%20of%20large%20structures.
- ↑ "New greenhouse gas identified". 11 March 2009. https://news.mit.edu/2009/prinn-greenhouse-tt0311.
- ↑ "Chapter 10 : Methyl Bromide". https://csl.noaa.gov/assessments/ozone/1994/chapters/chapter10.pdf.
- ↑ "Plant cyanogenic glycosides". Toxicon 38 (1): 11–36. January 2000. doi:10.1016/S0041-0101(99)00128-2. PMID 10669009.
- ↑ "Why are so many food plants cyanogenic?". Phytochemistry 47 (2): 155–162. January 1998. doi:10.1016/S0031-9422(97)00425-1. PMID 9431670. Bibcode: 1998PChem..47..155J.
- ↑ "Are Apple Cores Poisonous?". The Naked Scientists. 26 September 2010. http://www.thenakedscientists.com/HTML/index.php?id=31&tx_naksciquestions_pi1%5BshowUid%5D=2737&cHash=69220df3a3.
- ↑ "The toxic effects of cassava (manihot esculenta grantz) diets on humans: a review". Veterinary and Human Toxicology 33 (3): 274–275. June 1991. PMID 1650055.
- ↑ "Cyanogenesis in cassava. The role of hydroxynitrile lyase in root cyanide production". Plant Physiology 116 (4): 1219–1225. April 1998. doi:10.1104/pp.116.4.1219. PMID 9536038.
- ↑ "Secretion of Benzaldehyde and Hydrogen Cyanide by the Millipede Pachydesmus crassicutis (Wood)". Science 138 (3539): 512–513. October 1962. doi:10.1126/science.138.3539.512. PMID 17753947. Bibcode: 1962Sci...138..512B.
- ↑ "Cyanogenesis in Arthropods: From Chemical Warfare to Nuptial Gifts". Insects 9 (2): 51. May 2018. doi:10.3390/insects9020051. PMID 29751568.
- ↑ "The neutral photochemistry of nitriles, amines and imines in the atmosphere of Titan". Icarus 247: 218–247. February 2015. doi:10.1016/j.icarus.2014.09.039. Bibcode: 2015Icar..247..218L. https://lirias.kuleuven.be/handle/123456789/486735.
- ↑ "INMS-derived composition of Titan's upper atmosphere: Analysis methods and model comparison". Planetary and Space Science 57 (14–15): 1895–1916. December 2009. doi:10.1016/j.pss.2009.06.016. Bibcode: 2009P&SS...57.1895M.
- ↑ 38.0 38.1 "HCN Production in Titan's Atmosphere: Coupling Quantum Chemistry and Disequilibrium Atmospheric Modeling". Astrophysical Journal 901 (2): 110. 2020. doi:10.3847/1538-4357/abae5c. Bibcode: 2020ApJ...901..110P.
- ↑ "A Consistent Reduced Network for HCN Chemistry in Early Earth and Titan Atmospheres: Quantum Calculations of Reaction Rate Coefficients". The Journal of Physical Chemistry A 123 (9): 1861–1873. March 2019. doi:10.1021/acs.jpca.8b11323. PMID 30721064. Bibcode: 2019JPCA..123.1861P.
- ↑ "Making Sense of the Chemistry That Led to Life on Earth". The New York Times. 2015-05-04. https://www.nytimes.com/2015/05/05/science/making-sense-of-the-chemistry-that-led-to-life-on-earth.html.
- ↑ 41.0 41.1 "Hydrogen cyanide generation by mu-opiate receptor activation: possible neuromodulatory role of endogenous cyanide". Brain Research 768 (1–2): 294–300. September 1997. doi:10.1016/S0006-8993(97)00659-8. PMID 9369328.
- ↑ "Receptor mechanisms mediating cyanide generation in PC12 cells and rat brain". Neuroscience Research 49 (1): 13–18. May 2004. doi:10.1016/j.neures.2004.01.006. PMID 15099699.
- ↑ "Toxicology of some inorganic antihypertensive anions". Federation Proceedings 35 (1): 69–72. January 1976. PMID 1245233.
- ↑ "Hazardous compounds in tobacco smoke". International Journal of Environmental Research and Public Health 8 (2): 613–628. February 2011. doi:10.3390/ijerph8020613. PMID 21556207.
- ↑ Ruiz-Bermejo, Marta; Zorzano, María-Paz; Osuna-Esteban, Susana (2013). "Simple Organics and Biomonomers Identified in HCN Polymers: An Overview". Life 3 (3): 421–448. doi:10.3390/life3030421. PMID 25369814. Bibcode: 2013Life....3..421R.
- ↑ "The Chemistry of Diaminomaleonitrile and its Utility in Heterocyclic Synthesis". Tetrahedron 59 (16): 2749–2763. 2003. doi:10.1016/S0040-4020(03)00153-4.
- ↑ 47.0 47.1 "Observations of Radio Emission from Interstellar Hydrogen Cyanide". Astrophysical Journal 163: L47–L52. 1971. doi:10.1086/180664. Bibcode: 1971ApJ...163L..47S.
- ↑ "Cool Star Models". Molecules in Astrophysics: Probes and Processes. International Astronomical Union Symposia. Molecules in Astrophysics: Probes and Processes. 178. Springer Science & Business Media. 1997. p. 446. ISBN 978-0792345381. https://books.google.com/books?id=VW50otz5v8sC&pg=PA446.
- ↑ "Upper limits to trace constituents in Jupiter's atmosphere from an analysis of its 5-μm spectrum". Icarus 34 (2): 331–343. 1978. doi:10.1016/0019-1035(78)90171-9. Bibcode: 1978Icar...34..331T.
- ↑ "Submillimeter- and Millimeter-Wavelength Observations of SiO and HCN in Circumstellar Envelopes of AGB Stars". Astrophysical Journal 543 (2): 897–921. 2000. doi:10.1086/317129. Bibcode: 2000ApJ...543..897B.
- ↑ "Detection of a Second, Strong Sub-millimeter HCN Laser Line toward Carbon Stars". Astrophysical Journal 583 (1): 446–450. 2003. doi:10.1086/345099. Bibcode: 2003ApJ...583..446S.
- ↑ 52.0 52.1 "CN and HCN in Dense Interstellar Clouds". Astrophysical Journal 632 (1): 302–315. 2005. doi:10.1086/432864. Bibcode: 2005ApJ...632..302B.
- ↑ "The Star Formation Rate and Dense Molecular Gas in Galaxies". Astrophysical Journal 606 (1): 271–290. 2004. doi:10.1086/382999. Bibcode: 2004ApJ...606..271G.
- ↑ "HCN Survey of Normal Spiral, Infrared-luminous, and Ultraluminous Galaxies". Astrophysical Journal Supplement Series 152 (1): 63–80. 2004. doi:10.1086/383003. Bibcode: 2004ApJS..152...63G.
- ↑ "Indications of Inflow Motions in Regions Forming Massive Stars". Astrophysical Journal 592 (2): L79–L82. 2003. doi:10.1086/377679. Bibcode: 2003ApJ...592L..79W.
- ↑ "Molecular properties of (U)LIRGs: CO, HCN, HNC and HCO+". Proceedings IAU Symposium 242: 462–466. 2007. doi:10.1017/S1743921307013609. Bibcode: 2007IAUS..242..462L.
- ↑ "Release 14-038 – NASA's 3-D Study of Comets Reveals Chemical Factory at Work". NASA. 11 August 2014. http://www.nasa.gov/press/2014/august/goddard/nasa-s-3-d-study-of-comets-reveals-chemical-factory-at-work.
- ↑ "Mapping the Release of Volatiles in the Inner Comae of Comets C/2012 F6 (Lemmon) and C/2012 S1 (ISON) Using the Atacama Large Millimeter/Submillimeter Array". The Astrophysical Journal 792 (1): L2. 11 August 2014. doi:10.1088/2041-8205/792/1/L2. Bibcode: 2014ApJ...792L...2C.
- ↑ "First detection of super-earth atmosphere". ESA/Hubble Information Centre. February 16, 2016. https://phys.org/news/2016-02-super-earth-atmosphere.html.
- ↑ Green, Jaime (5 December 2023). "What Is Life? - The answer matters in space exploration. But we still don't really know.". The Atlantic. Archived from the original on 5 December 2023. https://archive.today/20231205121742/https://www.theatlantic.com/science/archive/2023/12/defining-life-existentialism-scientific-theory/676238/. Retrieved 15 December 2023.
- ↑ Chang, Kenneth (14 December 2023). "Poison Gas Hints at Potential for Life on an Ocean Moon of Saturn - A researcher who has studied the icy world said "the prospects for the development of life are getting better and better on Enceladus."". The New York Times. Archived from the original on 14 December 2023. https://archive.today/20231214210144/https://www.nytimes.com/2023/12/14/science/enceladus-moon-cyanide-life-saturn.html. Retrieved 15 December 2023.
- ↑ Peter, Jonah S. (14 December 2023). "Detection of HCN and diverse redox chemistry in the plume of Enceladus". Nature Astronomy: 1–10. doi:10.1038/s41550-023-02160-0. Bibcode: 2023NatAs.tmp..259P. Archived from the original on 15 December 2023. https://archive.today/20231215144349/https://www.nature.com/articles/s41550-023-02160-0. Retrieved 15 December 2023.
- ↑ Schnedlitz, Markus (2008) Chemische Kampfstoffe: Geschichte, Eigenschaften, Wirkung. GRIN Verlag. p. 13. ISBN:3640233603.
- ↑ Weapons of War - Poison Gas. firstworldwar.com
- ↑ 65.0 65.1 Environmental and Health Effects . Cyanidecode.org. Retrieved on 2012-06-02.
- ↑ "Hydrogen Cyanide". Organisation for the Prohibition of Chemical Weapons. http://www.opcw.org/about-chemical-weapons/types-of-chemical-agent/blood-agents/hydrogen-cyanide/.
- ↑ Van Pelt, Robert Jan; Dwork, Debórah (1996). Auschwitz, 1270 to the present. Norton. p. 443. ISBN 9780300067552. https://archive.org/details/auschwitz1270top00dwor/page/443.
- ↑ "Blue Fume". Chemical Factory Draslovka a.s.. https://www.draslovka.cz/about-us#products.
- ↑ "Ursgan D2". 2015-07-17. http://www.draslovka.cz/uragan-d2.
- ↑ Binkov's Battlegrounds (April 27, 2022). "How would have WW2 gone if the US had not used nuclear bombs on Japan?". https://www.youtube.com/watch?v=8CFPaSH84ZU.
- ↑ Pilkington, Ed (28 May 2021). "Arizona 'refurbishes' its gas chamber to prepare for executions, documents reveal". The Guardian. https://www.theguardian.com/us-news/2021/may/28/arizona-gas-chamber-executions-documents.
- ↑ "The Poison Garden website". http://thepoisongarden.co.uk/atoz/prunus_laurocerasus.htm.
- ↑ "Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs) – 74908". NIOSH. 2 November 2018. https://www.cdc.gov/niosh/idlh/74908.html.
External links
- Institut national de recherche et de sécurité (1997). "Cyanure d'hydrogène et solutions aqueuses". Fiche toxicologique n° 4, Paris:INRS, 5pp. (PDF file, in French)
- International Chemical Safety Card 0492
- Hydrogen cyanide and cyanides (CICAD 61)
- National Pollutant Inventory: Cyanide compounds fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- Department of health review
- Density of Hydrogen Cyanide gas
 |





