Chemistry:Germanium dichloride dioxane
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H8Cl2GeO2 | |
| Molar mass | 231.64 g·mol−1 |
| Appearance | white solid |
| Density | 1.942 g/cm3 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H314, H332, H351 | |
| P203Script error: No such module "Preview warning".Category:GHS errors, P260, P261, P264, P271, P280, P301+330+331, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P318Script error: No such module "Preview warning".Category:GHS errors, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
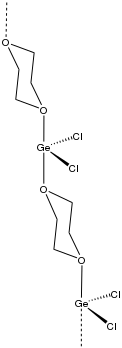
Germanium dichloride dioxane is a chemical compound with the formula GeCl
2(C
4H
8O
2), where C
4H
8O
2 is 1,4-dioxane. It is a white solid. The compound is notable as a source of Ge(II), which contrasts with the pervasiveness of Ge(IV) compounds. This dioxane complex represents a well-behaved form of germanium dichloride.
Synthesis and structure
It is prepared by reduction of a dioxane solution of germanium tetrachloride with tributyltin hydride:[2]
- GeCl
4 + 2 Bu
3SnH + C
4H
8O
2 → GeCl
2(O
2C
4H
8) + 2 Bu
3SnCl + H
2
Hydrosilanes have also been used as reductants.[3]
The complex has a polymeric structure. Germanium adopts an SF4-like shape with cis Cl ligands (Cl-Ge-Cl angle = 94.4°) and axial positions occupied by oxygen provided by a bridging dioxane. The Ge-O and Ge-Cl distances are 2.40 and 2.277 A, respectively.[4]
Reactions
The complex is used in the preparation of organogermanium compounds.[5][6] In organic synthesis, the complex is used as a Lewis acid with reducing properties.[3]
References
- ↑ "Germanium(II) chloride dioxane complex (1:1)" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/10857305#section=Safety-and-Hazards.
- ↑ Fjeldberg, Torgny; Haaland, Arne; Schilling, Birgitte E. R.; Lappert, Michael F.; Thorne, Andrew J. (1986). "Subvalent Group 4B Metal Alkyls and Amides. Part 8. Germanium and Tin Carbene Analogues MR2[M = Ge or Sn, R = CH(SiMe3)2]: Syntheses and Structures in the Gas Phase (Electron Diffraction); Molecular-Orbital Calculations for MH2 and GeMe2". Journal of the Chemical Society, Dalton Transactions (8): 1551. doi:10.1039/DT9860001551.
- ↑ 3.0 3.1 Roskamp, Carrie A.; Roskamp, Eric J. (2001). "Germanium Dichloride-Dioxane Complex". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rg002. ISBN 0471936235.
- ↑ Leites, L.A.; Zabula, A.V.; Bukalov, S.S.; Korlyukov, A.A.; Koroteev, P.S.; Maslennikova, O.S.; Egorov, M.P.; Nefedov, O.M. (2005). "Experimental and Theoretical Study of Vibrational Spectra and Structure of Dihalogermylene and Dihalostannylene Complexes with 1,4-Dioxane and Triphenylphosphine". Journal of Molecular Structure 750 (1–3): 116–122. doi:10.1016/j.molstruc.2005.04.015. Bibcode: 2005JMoSt.750..116L.
- ↑ Simons, Richard S.; Pu, Lihung; Olmstead, Marilyn M.; Power, Philip P. (1997). "Synthesis and Characterization of the Monomeric Diaryls M{C6H3-2,6-Mes2}2 (M = Ge, Sn, or Pb; Mes = 2,4,6-Me3C6H2−) and Dimeric Aryl−Metal Chlorides [M(Cl){C6H3-2,6-Mes2}]2 (M = Ge or Sn)". Organometallics 16 (9): 1920–1925. doi:10.1021/OM960929L.
- ↑ Roskamp, Carrie A.; Roskamp, Eric J. (2001). "Germanium Dichloride-Dioxane Complex". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rg002. ISBN 0471936235.
 |

