Chemistry:Krypton tetrafluoride
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| F4Kr | |
| Molar mass | 159.792 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Krypton(IV) fluoride is a hypothetical inorganic chemical compound of krypton and fluorine with the chemical formula KrF
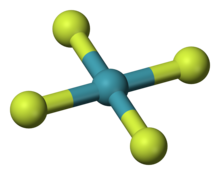
4. At one time researchers thought they had synthesized it, but the claim was discredited.[1] The compound is predicted to be difficult to make and unstable if made.[2] Theoretical analysis indicates KrF
4 would have an approximately square planar molecular geometry.[2]
Synthesis
The claimed synthesis was by passing electric discharge through krypton-fluorine mixture:[3]
- Kr + 2F
2 → KrF
4
- Kr + 2F
Physical properties
The claimed compound formed white crystalline solid.[4] Thermally, it is less stable than XeF
4.[5]
References
- ↑ O'Donnell, T. A. (8 June 2017) (in en). The Chemistry of Fluorine: Comprehensive Inorganic Chemistry. Elsevier. p. 1026. ISBN 978-1-4831-4642-3. https://books.google.com/books?id=ED79BAAAQBAJ&dq=Krypton+tetrafluoride&pg=PA1026. Retrieved 28 March 2023.
- ↑ 2.0 2.1 Dixon, David A.; Wang, Tsang-Hsiu; Grant, Daniel J.; Peterson, Kirk A.; Christe, Karl O.; Schrobilgen, Gary J. (2007). "Heats of Formation of Krypton Fluorides and Stability Predictions for KrF4 and KrF6 from High Level Electronic Structure Calculations". Inorg. Chem. 46 (23): 10016–10021. doi:10.1021/ic701313h. PMID 17941630.
- ↑ (in en) Advanced Inorganic Chemistry Vol-1. Krishna Prakashan Media. p. 846. ISBN 978-81-87224-03-7. https://books.google.com/books?id=0uwDTrxyaB8C&dq=Krypton+tetrafluoride&pg=PA846. Retrieved 28 March 2023.
- ↑ Cotton, F. Albert (1964) (in en). Progress in Inorganic Chemistry, Volume 6. John Wiley & Sons. p. 260. ISBN 978-0-470-16657-4. https://books.google.com/books?id=IhkQIoEGL24C&dq=Krypton+tetrafluoride&pg=PA260. Retrieved 28 March 2023.
- ↑ Grosse, A. V.; Kirshenbaum, A. D.; Streng, A. G.; Streng, L. V. (15 March 1963). "Krypton Tetrafluoride: Preparation and Some Properties". Science 139 (3559): 1047–1048. doi:10.1126/science.139.3559.1047. PMID 17812982. Bibcode: 1963Sci...139.1047G. https://www.science.org/doi/10.1126/science.139.3559.1047. Retrieved 28 March 2023.
 |

