Chemistry:Hexafluorophosphoric acid
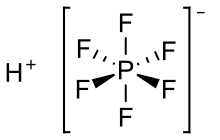
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hexafluorophosphoric acid[2]
| |||
| Other names
Hydrogen hexafluorophosphate
Hydron hexafluorophosphate | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| HPF6 | |||
| Molar mass | 145.972 g/mol | ||
| Appearance | colorless oily liquid | ||
| Melting point | decomposes at 25 °C | ||
| exists only in solution | |||
| Hazards | |||
| Main hazards | Corrosive | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H301, H311, H314, H330 | |||
| P260, P264, P271, P280, P284, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P320, P321, P363, P403+233, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Hexafluorophosphoric acid refers to a family of salts produced by combining phosphorus pentafluoride and hydrofluoric acid. The idealized chemical formula for hexafluorophosphoric acid
is HPF
6, which also is written H[PF]
6.[3] Hexafluorophosphoric acid is only stable in solution, decomposing to HF and PF5 when dry.[4] It exothermically reacts with water to produce oxonium hexafluorophosphate (H
3OPF
6) and hydrofluoric acid. Additionally, such solutions often contain products derived from hydrolysis of the P-F bonds, including HPO2F2, H2PO2F, and H3PO4, and their conjugate bases.[5] Hexafluorophosphoric acid attacks glass. Upon heating, it decomposes to generate HF. Crystalline HPF6 has been obtained as the hexahydrate, wherein PF−6 is enclosed in truncated octahedral cages defined by the water and protons. NMR spectroscopy indicates that solutions derived from this hexahydrate contain significant amounts of HF.[5]
Whereas a species with the formula HPF6 remains unknown, the analogous molecular hexafluoroarsenic acid (HAsF6) has been crystallized.[6]
See also
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–74. ISBN 0-8493-0594-2.
- ↑ IUPAC. "Nomenclature of Inorganic Chemistry". https://moam.info/nomenclature-of-inorganic-chemistry-iupac_59c16afb1723ddd2fb171db1.html.
- ↑ Arpad Molnar; G. K. Surya Prakash; Jean Sommer (2009). Superacid Chemistry (2nd ed.). Wiley-Interscience. p. 44. ISBN 978-0-471-59668-4.
- ↑ Lindahl., Charles B.; Mahmood, Tariq (2000), "Fluorine compounds, inorganic, phosphorus", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961.1608151912091404.a01, ISBN 9780471238966, http://onlinelibrary.wiley.com/book/10.1002/0471238961
- ↑ 5.0 5.1 D. W. Davidson; S. K. Garg (May 1972). "The Hydrate of Hexafluorophosphoric Acid". Canadian Journal of Chemistry 50 (21): 3515–3520. doi:10.1139/v72-565.
- ↑ Axhausen, Joachim; Lux, Karin; Kornath, Andreas (2014). "The Existence of Hexafluoroarsenic(V) Acid". Angewandte Chemie International Edition 53 (14): 3720–3721. doi:10.1002/anie.201308023. PMID 24446235.
 |