Chemistry:Iodosyl trifluoride
From HandWiki
| |
| |
| Names | |
|---|---|
| Other names
Iodine oxide trifluoride, iodosyltrifluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
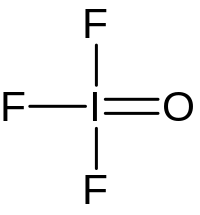
| F3IO | |
| Molar mass | 199.899 g·mol−1 |
| Appearance | colorless needles |
| Density | 3.95 g/cm3 |
| Related compounds | |
Related compounds
|
Chlorosyl trifluoride Iodosyl pentafluoride Bromosyl trifluoride Iodyl fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Iodosyl trifluoride is an inorganic compound of iodine, fluorine, and oxygen with the chemical formula IOF
3.[1]
Synthesis
Synthesis of iodosyl trifluoride can be done by a reaction of iodine pentoxide with iodine pentafluoride.[2]
- I
2O
5 + 3IF
5 → 5IOF
3
- I
Synthesis can be by a reaction of gases:[3]
- I
2 + O
2 + 3F
2 → 2IOF
3
- I
Or alternately by reaction of iodine pentafluoride with water:
- IF
5 + H
2O → IOF
3 + 2HF
- IF
Physical properties
Iodosyl trifluoride forms hygroscopic colorless needles. Reacts with water.[4]
Chemical properties
Iodosyl trifluoride is hygroscopic and decomposes into IO
2F and IF
5 at 110 °C.[5]
References
- ↑ Viers, Jimmy W.; Baird, H. Wallace (1 January 1967). "The crystal structure of iodine oxide trifluoride" (in en). Chemical Communications (21): 1093–1094. doi:10.1039/C19670001093. ISSN 0009-241X. https://pubs.rsc.org/en/content/articlelanding/1967/c1/c19670001093. Retrieved 24 May 2023.
- ↑ Wiberg, Egon; Wiberg, Nils (2001) (in en). Inorganic Chemistry. Academic Press. p. 468. ISBN 978-0-12-352651-9. https://books.google.com/books?id=Mtth5g59dEIC&dq=Iodosyl+trifluoride&pg=PA468. Retrieved 24 May 2023.
- ↑ Aynsley, E. E.; Nichols, R.; Robinson, P. L. (1 January 1953). "126. Reactions of iodine pentafluoride with inorganic substances. Iodine oxytrifluoride and iodyl fluoride" (in en). Journal of the Chemical Society: 623–626. doi:10.1039/JR9530000623. ISSN 0368-1769. https://pubs.rsc.org/en/content/articlelanding/1953/JR/jr9530000623. Retrieved 24 May 2023.
- ↑ Haynes, William M. (4 June 2014) (in en). CRC Handbook of Chemistry and Physics. CRC Press. pp. 4–67. ISBN 978-1-4822-0868-9. https://books.google.com/books?id=bNDMBQAAQBAJ&dq=Iodosyl+trifluoride&pg=SA4-PA67. Retrieved 24 May 2023.
- ↑ 第2版, 化学辞典. "ヨードシル塩(ヨードシルエン)とは? 意味や使い方" (in ja). https://kotobank.jp/word/%E3%83%A8%E3%83%BC%E3%83%89%E3%82%B7%E3%83%AB%E5%A1%A9-2123295.
 |

