Chemistry:Yttrium(III) fluoride
| |
| Names | |
|---|---|
| Other names
yttrium trifluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| YF3 | |
| Molar mass | 145.90 g mol−1 |
| Appearance | white powder |
| Density | 4.01 g cm−3 |
| Melting point | 1,387 °C (2,529 °F; 1,660 K) |
| Boiling point | 2,230 °C (4,050 °F; 2,500 K) |
| insoluble | |
| Solubility in acid | soluble |
Refractive index (nD)
|
1.51 (500 nm) |
| Structure | |
| Orthorhombic, oP16, SpaceGroup = Pnma, No. 62 | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P322, P330, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Yttrium(III) chloride Yttrium(III) bromide Yttrium(III) iodide |
Other cations
|
Scandium(III) fluoride Lutetium(III) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula YF3. It is not known naturally in 'pure' form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY(F,Cl)6. Sometimes mineral fluorite contains admixtures of yttrium.[1][2]
Synthesis
YF3 can be produced by reacting fluorine with yttria or yttrium hydroxide with hydrofluoric acid.[3]
- Y(OH)3 + 3HF → YF3 + 3H2O
Properties
Yttrium(III) fluoride has a refractive index of 1.51 at 500 nm[4] and is transparent in the range from 193 nm to 14,000 nm (i.e. from the UV to IR range).
Pure yttrium can be obtained from yttrium(III) fluoride by reduction with calcium.
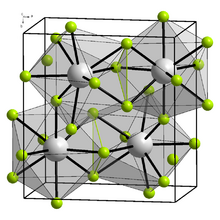
Yttrium(III) fluoride crystallizes in the orthorhombic crystal system, with space group Pnma (space group no. 62), with the lattice parameters a = 6.3537 Å, b = 6.8545 Å, c = 4.3953 Å.[5] Yttrium is nine times coordinated by fluorine atoms.
Occurrence and uses
It occurs as the mineral waimirite-(Y).[6]
Yttrium(III) fluoride can be used for the production of metallic yttrium,[7] thin films, glasses[8] and ceramics.
Hazards
Conditions/substances to avoid are: acids, active metals and moisture.
References
- ↑ Dinér, Peter (February 2016). "Yttrium from Ytterby" (in en). Nature Chemistry 8 (2): 192. doi:10.1038/nchem.2442. ISSN 1755-4349. PMID 26791904.
- ↑ "Tiny particles produce huge photon avalanches" (in en-GB). 2021-01-21. https://physicsworld.com/tiny-particles-produce-huge-photon-avalanches/.
- ↑ SCHENK, P.W.; BRAUER, G. (1963), "Preparative Methods", Handbook of Preparative Inorganic Chemistry (Elsevier): pp. 3–107, http://dx.doi.org/10.1016/b978-0-12-395590-6.50008-9, retrieved 2023-12-24
- ↑ "General Reserch Institute Nonferrous Metals". 2007-09-28. https://web.archive.org/web/20070928110301/http://en.grinm.com/channel.do?cmd=preview&id=348&&nid=1254.
- ↑ Cheetham, A. K.; Norman, N.; Hope, Håkon; Kjekshus, Arne; Klewe, Bernt; Powell, D. L. (1974). "The Structures of Yttrium and Bismuth Trifluorides by Neutron Diffraction.". Acta Chemica Scandinavica 28a: 55–60. doi:10.3891/acta.chem.scand.28a-0055. ISSN 0904-213X. http://dx.doi.org/10.3891/acta.chem.scand.28a-0055.
- ↑ "Waimirite-(Y): Mineral information, data and localities.". https://www.mindat.org/min-46049.html.
- ↑ "Wayback Machine". https://web.archive.org/web/20100705064306/http://www.cerac.com/pubs/proddata/yf3.pdf.
- ↑ "Yttrium Fluoride 99%-99.999% from Metall Rare Earth Limited". http://www.metall.com.cn/yf.htm.
 |
