Chemistry:Niobium(III) chloride
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Cl3Nb | |
| Molar mass | 199.26 g·mol−1 |
| Appearance | black solid |
| Density | 3.75 |
| Structure[1] | |
| hexagonal | |
| P3m1 | |
a = 6.744, c = 12.268
| |
Formula units (Z)
|
2 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Niobium(III) chloride also known as niobium trichloride is a compound of niobium and chlorine. The binary phase NbCl3 is not well characterized but many adducts are known.
Synthesis
thumb|left|184px|Structure of NbCl3(dimethoxyethane)(3-hexyne).[2] Nb3Cl8 is produced by reduction of niobium(V) chloride with hydrogen, or just by heating.
Salt-free reduction of dimethoxyethane solution of NbCl5 with 1,4-disilyl-cyclohexadiene in the presence of 3-hexyne produces the coordination complex NbCl3(dimethoxyethane)(3-hexyne):
- NbCl5 + C6H6(SiMe3)2 + C2Et2 + dme → NbCl3(dme)(C2Et2) + C6H6 + 2 Me3SiCl
An impure dimethoxyethane (dme) adduct of niobium trichloride was produced by reduction of a dme solution of niobium pentachloride with tributyltin hydride:[3]
- NbCl5 + 2 Bu3SnH + MeOCH2CH2OMe → NbCl3(MeOCH2CH2OMe) + 2 Bu3SnCl
Structure
Nb3Cl8 has a hexagonal close packed array of chloride ions. Triangles of niobium occur in octahedral spaces in the chloride array. The compositions with higher chloride have some niobium atoms missing from the structure, creating vacancies and giving rise to nonstoichiometric compounds. NbCl4 has this pattern of vacancies stretched until the niobium atoms are in pairs rather than triangles. So NbCl3 can be considered as a solid solution of Nb3Cl8 and Nb2Cl8.[4]
The colour of niobium trichloride varies depending on the niobium:chloride ratio. NbCl2.67 is green, while NbCl3.13 is brown.[1]
Reactions
When heated to over 600 °C niobium trichloride disproportionates to niobium metal and niobium pentachloride.
Adducts
NbCl3(dimethoxyethane) has received significant attention as a reagent for reductive coupling of carbonyls and imines.[6] It is sold as a 1,2-dimethoxyethane complex. Nb(III) adducts are also known for 1,4-dioxane and diethyl ether.
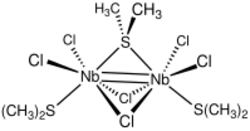
Niobium(III) chloride forms a series of compounds with the formula Nb2Cl6Lx with Nb=Nb double bond. With tertiary phosphines and arsines, the complexes are edge-share bioctahedra, e.g., Nb2Cl6(PPhMe2)4.[7] Thioethers form adducts with one bridging thioether (R2S). These face-sharing bioctahedra have the formula Nb2X6(R2S)3 (X = Cl, Br).
References
- ↑ 1.0 1.1 Gutmann, Viktor (1967) (in en). Halogen Chemistry. Elsevier. p. 157. ISBN 978-0-323-14847-4. https://books.google.com/books?id=q0j8Imd8yQsC&pg=PA157.
- ↑ Tsurugi, Hayato; Mashima, Kazushi (2019). "Salt-Free Reduction of Transition Metal Complexes by Bis(trimethylsilyl)cyclohexadiene, -dihydropyrazine, and -4,4′-bipyridinylidene Derivatives". Accounts of Chemical Research 52 (3): 769–779. doi:10.1021/acs.accounts.8b00638. PMID 30794373.
- ↑ Pedersen, Steven F.; Hartung, Jack B.; Roskamp, Eric J.; Dragovich, Peter S. (1992). "Niobium(III) and (IV) Halide Complexes". Inorganic Syntheses. 28. pp. 119–123. doi:10.1002/9780470132609.ch28. ISBN 9780470132609.
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey (1966). Advanced Inorganic Chemistry: A Comprehensive Text. John Wiley. p. 927.
- ↑ Kakeya, Masaki; Fujihara, Takashi; Nagasawa, Akira (2006). "Di-μ-chloro-μ-(dimethyl sulfide)-bis[dichloro(dimethyl sulfide)niobium(III)]". Acta Crystallographica Section E 62 (3): m553–m554. doi:10.1107/S1600536806005149. Bibcode: 2006AcCrE..62M.553K.
- ↑ Roskamp, Carrie A.; Roskamp, Eric J. (2001). "Encyclopedia of Reagents for Organic Synthesis". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rt202s. ISBN 0471936235.
- ↑ Sharma, Sangeeta; Vermani, O. P.; Narula, A. K. (January 1996). "Synthesis and Structural Studies of Complexes of Niobium(III) Chloride with Ditertiary Phosphines". Indian Journal of Chemistry, Section A 35A (1). ISSN 0975-0975. http://nopr.niscair.res.in/handle/123456789/41226.
 |

