Chemistry:Cholesterol sulfate
From HandWiki
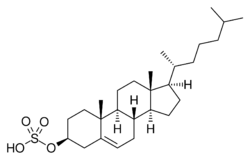
| |
| Names | |
|---|---|
| IUPAC name
[(3S,8S,9S,10R,13R,14S,17R)-10,13-Dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] hydrogen sulfate
| |
| Other names
Cholest-5-en-3β-ol sulfate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H46O4S | |
| Molar mass | 466.72 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Cholesterol sulfate, or cholest-5-en-3β-ol sulfate, is an endogenous steroid and the C3β sulfate ester of cholesterol.[1][2] It is formed from cholesterol by steroid sulfotransferases (SSTs) such as SULT2B1b (also known as cholesterol sulfotransferase)[2] and is converted back into cholesterol by steroid sulfatase (STS).[1] Accumulation of cholesterol sulfate in the skin is implicated in the pathophysiology of X-linked ichthyosis, a congenital disorder in which STS is non-functional and the body cannot convert cholesterol sulfate back into cholesterol.[1][2]
See also
References
- ↑ 1.0 1.1 1.2 Peter M. Elias (21 January 2016). Advances in Lipid Research: Skin Lipids. Elsevier. pp. 45–46. ISBN 978-1-4832-1545-7. https://books.google.com/books?id=rTeaBQAAQBAJ&pg=PA45.
- ↑ 2.0 2.1 2.2 P. Itin; G. Jemec (15 September 2010). Ichthyoses. Karger Medical and Scientific Publishers. pp. 59–. ISBN 978-3-8055-9395-3. https://books.google.com/books?id=paw6AQAAQBAJ&pg=PA59.
 |

