Chemistry:Rapacuronium bromide
From HandWiki
Short description: Pharmaceutical drug
This article relies largely or entirely on a single source. (November 2007) |
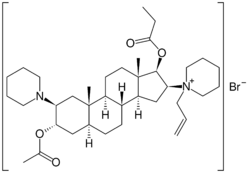 Rapacuronium bromide | |
| Clinical data | |
|---|---|
| Other names | [(2S, 3S, 5S, 8R, 9S, 10S, 13S, 14S, 16S, 17S)-3-acetyloxy-10,13-dimethyl-2-(1-piperidyl)-16-(1-prop-2-enyl-3,4,5,6-tetrahydro-2H-pyridin-1-yl)-2 ,3 ,4 ,5 ,6 ,7 ,8 ,9 ,11 ,12 ,14, 15, 16, 17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]propanoate |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Not applicable |
| Protein binding | Variable |
| Metabolism | Hydrolyzed to active metabolites CYP system not involved |
| Elimination half-life | 141 minutes (mean) |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C37H61N2O4+ |
| Molar mass | 597.905 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Rapacuronium bromide (brand name Raplon) is a rapidly acting, non-depolarizing aminosteroid neuromuscular blocker formerly used in modern anaesthesia, to aid and enable endotracheal intubation, which is often necessary to assist in the controlled ventilation of unconscious patients during surgery and sometimes in intensive care. As a non-depolarizing agent it did not cause initial stimulation of muscles before weakening them.[1]
Due to risk of fatal bronchospasm it was withdrawn from the United States market by Organon on March 27, 2001, less than 2 years after its FDA approval in 1999.[2]
References
- ↑ "Rapacuronium bromide: a review of its use in anaesthetic practice". Drugs 58 (5): 887–918. November 1999. doi:10.2165/00003495-199958050-00011. PMID 10595867.
- ↑ "Voluntary Market Withdrawal". Food and Drug Administration. 27 March 2001. https://www.fda.gov/medwatch/SAFETY/2001/raplon_DDR.pdf.
 |

