Chemistry:Gallium(III) fluoride
From HandWiki
| |

| |
| Names | |
|---|---|
| Other names
gallium trifluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| GaF3 | |
| Molar mass | 126.718 g/mol |
| Appearance | white powder |
| Density | 4.47 g/cm3 |
| Melting point | 800 °C (1,470 °F; 1,070 K) |
| Boiling point | 1,000 °C (1,830 °F; 1,270 K) |
| 0.0002 g/100 mL | |
| Structure | |
| Rhombohedral, hR24 | |
| R-3c, No. 167 | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H312, H332 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+312, P304+340, P312, P322, P330, P363, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
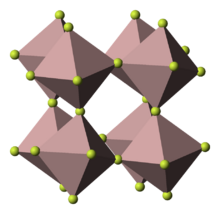
Gallium(III) fluoride (GaF3) is a chemical compound. It is a white solid that melts under pressure above 1000 °C but sublimes around 950 °C. It has the FeF3 structure where the gallium atoms are 6-coordinate.[1] GaF3 can be prepared by reacting F2 or HF with Ga2O3 or by thermal decomposition of (NH4)3GaF6.[2] GaF3 is virtually insoluble in water.[2] Solutions of GaF3 in HF can be evaporated to form the trihydrate, GaF3·3H2O, which on heating gives a hydrated form of GaF2(OH).[2] Gallium(III) fluoride reacts with mineral acids to form hydrofluoric acid.
 |
100px | 100px | |
| view along the a axis | view along the c axis | Ga coordination | F coordination |
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ 2.0 2.1 2.2 Anthony John Downs, (1993), Chemistry of Aluminium, Gallium, Indium, and Thallium, Springer, ISBN:978-0-7514-0103-5
Further reading
- Barrière, A.S.; Couturier, G.; Gevers, G.; Guégan, H.; Seguelond, T.; Thabti, A.; Bertault, D. (1989). "Preparation and characterization of gallium(III) fluoride thin films". Thin Solid Films 173 (2): 243. doi:10.1016/0040-6090(89)90140-5. Bibcode: 1989TSF...173..243B.
 |


