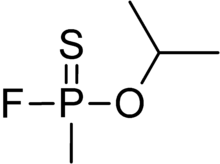
Chemistry:Thiosarin
| |
| Names | |
|---|---|
| Other names
O-Isopropyl methylphosphonofluoridothioate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C4H10FOPS | |
| Molar mass | 156.16 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Thiosarin, sulfursarin or GBS, is the organophosphorus compound analogous to sarin.[1] It differs structurally in that sulfur replaces the oxygen of the P=O bond. It is an extremely toxic substance related to G-agents.[2]
Characteristics
For thiosarin, unlike sarin, the literature contains little information.[3] It is reported as a colorless liquid with a characteristic organosulfur odor when pure. It is estimated to have a boiling point of 144-167 °C. It is a more nonpolar compound, with a solubility in water of 7 g/L. Thiosarin probably belongs to the IVA compound series, leaving it much less volatile than sarin. It has a greater persistence in the environment than sarin.[2]
Absorption frequencies of sarin derivatives showed that the frequency of stretching of the P-F and P=S bond of thiosarin is lower than that of its oxygenated analogue.[4]
CW history and candidate
Its toxicity was discovered in the 1970s by Friedrick Wilhelm Hoffmann and Ray King Irino. They were responsible for synthesizing and analyzing a series of sulfur G-agent compounds.[5] The open literature reports that the compound has been cataloged as GS, but this statement is incorrect, it belongs to EA-1246. GS agents are a series of G compounds. GBS is generally lower than sarin.[5] The little open military literature may be due to the low toxicity of this series of compounds.
The possibility of a chemical warfare agent candidate was raised when Bogomazov and his colleagues discovered that thiosarin had the ability to break through military gas mask filters, where it would then be converted to its analogue.[6]:115</ref> An investigation by Vil Mirzayanov refuted these results.[6]:118
Thiosarin is used as a precursor to sarin.
Synthesis
The preparation route is quite similar to that of sarin.[5] The synthesis routes of thiosarin are manifold.[7][1]
Regardless of the synthesis route chosen, the final reaction is usually the reaction of isopropyl methylthiophosphonochloridate with fluorides.[2]
Reactions
Thiosarin has a tendency to convert to its analogue by different mechanisms. In anhydrous medium, thiosarin is oxidized to form GB.[8]
In the controlated aqueous medium, without the presence of oxygen, the tendency is to evolve hydrogen sulfide.
Sulfur is more nucleophilic and tends to form the thiolate isomer at ~100 °C.[9]
Sarin-S
Along with the discovery of the high toxicity of this series of compounds, Hoffmann discovered that the S-alkyl isomers, unlike the alkyl alkylphosphonothiol compounds, were less toxic than the G(S) agents.[5]
References
- ↑ 1.0 1.1 U.S. Chemical Warfare Policy: Hearings, Ninety-third Congress, Second Session. May 1, 2, 7, 9 and 14, 1974. pg 341-344
- ↑ 2.0 2.1 2.2 Ledgard, J. A Laboratory History of Chemical Warfare Agents. 171-174
- ↑ Augerson, W. S. Review of the Scientific Literature as it Pertains to Gulf War Illnesses. Volume 5. Chemical and Biological Warfare Agents. p 200
- ↑ Theodorus., Kuiper, Antonius Emilius (1974). Adsorption and decomposition of sarin on gamma-alumina. OCLC 634367125. http://worldcat.org/oclc/634367125.
- ↑ 5.0 5.1 5.2 5.3 Hoffmann et al. US 4,012,465
- ↑ 6.0 6.1 S., Mirzayanov, Vil (2009). State secrets : an insider's chronicle of the Russian chemical weapons program. Outskirts Press, Inc. ISBN 978-1-4327-2566-2. OCLC 299069417. http://worldcat.org/oclc/299069417.
- ↑ Turkington, Robert (2010). Chemicals used for illegal purposes : a guide for first responders to identify explosives, recreational drugs, and poisons. Wiley. ISBN 978-0-470-18780-7. OCLC 489624232. http://worldcat.org/oclc/489624232.
- ↑ Tetrahedron Letters, Vo1, .28, No.26, pp 2981-2984, 1987
- ↑ Senkbeil et al. US 3,337,658
 |

