Chemistry:Theacrine
| |
| |
| Names | |
|---|---|
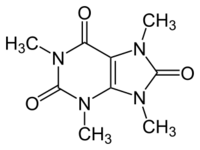
| Preferred IUPAC name
1,3,7,9-Tetramethyl-7,9-dihydro-1H-purine-2,6,8(3H)-trione | |
| Other names
1,3,7,9-Tetramethyluric acid; Temurin; Temorine; Tetramethyluric acid; Tetramethyl uric acid; TeaCrine (trade name)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H12N4O3 | |
| Molar mass | 224.220 g·mol−1 |
| Melting point | 226 °C (439 °F; 499 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
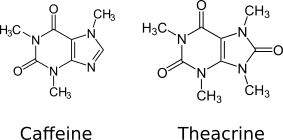
Theacrine, also known as 1,3,7,9-tetramethyluric acid, is a purine alkaloid found in Cupuaçu (Theobroma grandiflorum) and in a Chinese tea known as kucha (Chinese: 苦茶; pinyin: kǔ chá; literally: 'bitter tea') (Camellia assamica var. Kucha).[1][2] It shows anti-inflammatory and analgesic effects and appears to affect adenosine signalling in a manner similar to caffeine.[2][3] In kucha leaves, theacrine is synthesized from caffeine in what is thought to be a three-step pathway.[2] Theacrine and caffeine are structurally similar.
Pharmacology
Pharmacodynamics
The exact mechanism of action of theacrine is uncertain, as no binding affinities have been published. However, animal research involving selective A1 and A2A adenosine antagonists indicates that theacrine is likely an adenosine antagonist.[2]
Administration of selective dopamine D1 and D2 antagonists demonstrate that, similarly to caffeine,[4] theacrine's actions are in part mediated by dopamine receptors.[2] This should not be taken as evidence that theacrine directly interacts with dopamine receptors, as some marketers have misleadingly claimed.[citation needed]
Pharmacokinetics
Theacrine has half-life of 30 to 33 hours.[5]
Safety
Theacrine has demonstrated clinical safety and non-habituating effects in healthy humans over eight weeks of daily use at up to 300 mg/day.[6] Moreover, there was no evidence of the tachyphylaxis typical of neuroactive agents like caffeine and other stimulants.[6]
In animal studies, theacrine has an LD50 of 810 mg/kg,[3][6] compared to 265 mg/kg for caffeine.[7]
See also
References
- ↑ Zheng, XQ; Ye, CX; Kato, M; Crozier, A; Ashihara, H (2002). "Theacrine (1,3,7,9-tetramethyluric acid) synthesis in leaves of a Chinese tea, kucha (Camellia assamica var. Kucha)". Phytochemistry 60 (2): 129–34. doi:10.1016/s0031-9422(02)00086-9. PMID 12009315.
- ↑ 2.0 2.1 2.2 2.3 2.4 Feduccia, Allison A.; Wang, Yuanyuan; Simms, Jeffrey A.; Yi, Henry Y.; Li, Rui; Bjeldanes, Leonard; Ye, Chuangxing; Bartlett, Selena E. (2012). "Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: Involvement of adenosine and dopamine receptors". Pharmacology Biochemistry and Behavior 102 (2): 241–248. doi:10.1016/j.pbb.2012.04.014. PMID 22579816.
- ↑ 3.0 3.1 Wang, Yuanyuan; Yang, Xiaorong; Zheng, Xinqiang; Li, Jing; Ye, Chuangxing; Song, Xiaohong (2010). "Theacrine, a purine alkaloid with anti-inflammatory and analgesic activities". Fitoterapia 81 (6): 627–631. doi:10.1016/j.fitote.2010.03.008. PMID 20227468.
- ↑ Garrett, B. E.; Holtzman, S. G. (January 1994). "D1 and D2 dopamine receptor antagonists block caffeine-induced stimulation of locomotor activity in rats". Pharmacology, Biochemistry, and Behavior 47 (1): 89–94. doi:10.1016/0091-3057(94)90115-5. ISSN 0091-3057. PMID 7906891. https://pubmed.ncbi.nlm.nih.gov/7906891/.
- ↑ Mondal, Goutam; Wang, Yan-Hong; Butawan, Matthew; Bloomer, Richard J; Yates, Ryan (2021-01-06) (in en). Caffeine and Methylliberine: A Human Pharmacokinetic Interaction Study (Report). Pharmacology and Therapeutics. doi:10.1101/2021.01.05.21249234. http://medrxiv.org/lookup/doi/10.1101/2021.01.05.21249234.
- ↑ 6.0 6.1 6.2 "Safety of TeaCrine®, a non-habituating, naturally-occurring purine alkaloid over eight weeks of continuous use". Journal of the International Society of Sports Nutrition 13: 2. 2016. doi:10.1186/s12970-016-0113-3. PMID 26766930.
- ↑ "Comparative toxicity of caffeine and aminophylline (theophylline ethylenediamine) in young and adult rats". Biology of the Neonate 34 (1–2): 68–71. 1978. doi:10.1159/000241107. PMID 698326.
 |